The interhospital transport of infants with persistent pulmonary hypertension of the newborn (PPHN) being administered inhaled nitric oxide (iNO) therapy requires specialized, intensive care. By understanding the logistical and safety challenges of the process, as well as the advantages that transport-friendly equipment can provide, clinicians can successfully sustain iNO delivery from departure to arrival.
By Natalie Mitchell, MBEi
Robert E. Newmyer, MDii,iii
Mary Cominek, RRT, C-NPTii
Rupa Crite, RRT, RRT-NPSi
Heloisa Georgiev, RRT-NPS, C-NPTi
Charles V. Pollack, Jr, MA, MDiv
Background: PPHN
The incidence of persistent pulmonary hypertension of the newborn (PPHN) in infants ≥34 weeks gestational age without congenital heart disease, is approximately 0.2% in the United States.1 A high-risk diagnosis that carries a one-year mortality rate of 7-8% and is often associated with serious congenital anomalies of the respiratory tract (in which patients one-year mortality may exceed 30%),1
PPHN requires intensive cardiopulmonary care. In severe cases or in those patients who fail to respond to supportive measures and specific treatment of associated lung disease, further interventions include the use of inhaled nitric oxide (iNO), a selective pulmonary vasodilator that reduces the ratio of pulmonary to systemic vascular resistance (PVR/SVR). With iNO, oxygenation improves as vessels are dilated in better-ventilated parts of the lung, and the need for extracorporeal membrane oxygenation (ECMO) to provide adequate tissue oxygenation is diminshed.2-3
Management of PPHN : When Emergency Transport Includes iNO
As is apparent from even this briefest of descriptions, management of PPHN requires specialized, intensive care. There are nearly 4,000 acute care hospitals in the US, and 262 designated children’s hospitals of varying sizes.4 There are about 750 neonatal intensive care units (NICUs) in the US, and of those, the American Academy of Pediatrics designates fewer than half as Level III or IV units. These are the sites of comprehensive care where ECMO and cardiovascular surgery that are appropriate to support iNO treatment of PPHN are available.5 This means that the 30-40% of newborns with severe hypoxemia due to pulmonary hypertension who show a suboptimal or nonsustained response to iNO6 must be transported with advanced life support (ALS) capability and ongoing administration of iNO from one hospital to an ECMO-capable center.
Abrupt discontinuation of iNO therapy before transport in patients who have not improved oxygenation and hemodynamics can be harmful because of acute deterioration with severe hypoxemia (“rebound” pulmonary hypertension). Therefore, the standard of care is to transport such patients while continuing iNO treatment.
In fact, initiation of iNO therapy at the referring hospital is associated with decreased length of hospitalization in those infants not ultimately requiring ECMO.7 While not part of the labeled indication for iNO, therapy may also be initiated prior to transport in the management of other illness accompanied by pulmonary hypertension, including bronchopulmonary dysplasia, meconium aspiration, pulmonary hemorrhage, pulmonary hypoplasia, tracheoesophageal fistula, transposition of the great vessels or other congenital heart defects, or sepsis with respiratory failure.6
In October 2020, Vero Biotech hosted an advisory board to discuss the primary objectives of patient transport, opportunities to improve quality of care associated with current iNO transport solutions, and inherent work flow challenges that could be resolved with more effective tools. The attendees, included respiratory therapists, flight nurses, and an air transport physician. This review reflects the key elements and learnings of their discussion.
Standards for Transport with iNO Systems
There are standards for interhospital transport of patients on iNO, promulgated in 2016 by the Association of Critical Care Transport.9 The basic components of those standards are:
- Blending gas capability including supplemental oxygen and room air
- Proper administration device that integrates with the ventilator used in transport
- Sufficient iNO capacity for the maximum duration of transport, plus a 30-minute reserve
- Temperature stabilization for the nitric oxide.
These standards are in addition to special controls set forth by the FDA in a guidance document entitled “Premarket Notification Submissions for Nitric Oxide Delivery Apparatus, Nitric Oxide Analyzer and Nitrogen Dioxide Analyzer.” The apparatus must allow reliable maintenance of an approximately constant concentration of iNO during inspiration, regardless of variation in flow rates, as set typically in the range of 0 to 80 parts per million (ppm). It must include a pressure regulator and connectors with fittings which are specific for nitric oxide gas cylinders, and must be designed to limit the time that NO is mixed with oxygen, thus minimizing the production of NO2.
Importantly, the delivery system must include a separate, redundant set-up for use as a “backup” system to supply iNO when the primary administration apparatus cannot be used.
The Commission on Accreditation of Medical Transport Systems (CAMTS) standards require that teams providing interfacility transport have the capability to deliver out-of-hospital care at a specialty or subspecialty level (eg, comparable to that of a tertiary or quaternary such as an ICU, PICU, NICU, or tertiary perinatal center). This includes the capability to provide blended gases, specifically citing iNO.10
As of early December 2020, no iNO Delivery System (NODS) in the US has FDA approval for transport that meets the current AACT and CAMTS standards, which can also be used in acute care. Additionally, the Federal Aviation Administration (FAA) has not developed a uniform policy to guide the use of iNO on fixed-wing or rotary-wing flights. Nonetheless, interhospital transport using iNO was offered as early as 1993,8 even though the FDA did not approve iNO for PPHN until 1999.
Currently accepted standard of care is that in the event of a suboptimal response to iNO, non-ECMO centers that offer iNO therapy to infants should have an iNO transport system readily and consistently available, and be prepared to initiate early referral.
What Options Are Currently Available for Transport with iNO?
The ideal NODS would be configured for use in both acute care and interhospital transport, which may occur by ground, rotary-wing, or fixed-wing mode. Transport of critically ill neonates on iNO therapy is a common occurrence at larger and regional children’s hospitals, ranging from once per month to many dozens per year.
The most utilized conventional ventilators for neonatal transports are the electronic Crossvent 2 i+ Infant Ventilator (Bio-Med Devices Inc) and the Hamilton T1 ventilator (Hamilton Medical). The pneumatic MVP-10 ventilator (Bio-Med Devices Inc) is sometimes also utilized. Nitric oxide may be delivered accurately via any of these ventilators. If high frequency ventilation (HFV) is needed, most NICU transport teams utilize the TXP series (Percussionaire Corp). The Bronchotron (Percussionaire Corp) is a dual ventilator that includes a conventional and a pneumatic HFV device. Mallinckrodt devices are not compatible with these HFV devices, leaving the transport team currently with the single option of the AeroNOx device (International Biomedical) in conjunction with Mallinckrodt NO tanks.
Logistics of Transport with iNO
Using multiple types of ventilators and NODS, from different manufacturers, results in an increase in costs to the hospital. This approach also creates more regular and intensive training requirements in order to keep staff credentialed on and comfortable with two or more NODS, including in the transport setting where working space is severely limited and when there are substantial differences regarding set-up and manual resuscitation process for different devices. This scenario increases the likelihood for error to occur in the transport of a critically ill patient. On the contrary, the potential to improve the quality of care associated with continuity of equipment use across the entire care path for the delivery of iNO via any conventional or high frequency ventilator during neonatal transports would streamline and improve this process.
In addition to that, training on multiple types of equipment for the current transport process necessitates that the team overcome important and frequently encountered logistical obstacles. With variable flow modes, for example, potentially wide fluctuations in flow require adjustment from breath to breath. Space restrictions occur in both ground and air transport vehicles, especially if additional support is needed for the patient, or if bagging is required. Bagging with currently used equipment requires a second NO tank with a specialized regulator. Use of other types of tank-based equipment requires reconfiguration of the transport apparatus; the patient must be bagged with an iNO blender, which must itself be mounted along with the delivery system monitor onto the transporter.
Both current systems require an additional tank to be added to the transporter configuration, or another tank must be removed for the iNO tank to be added. This can be an issue if ambulance or helicopter does not have onboard gas supplies for the ventilator and during long distance transports. In addition, weight is an issue for neonatal transports; the isolette itself weighs 300 lbs or more before the NODS is added. This total weight makes it more difficult, particularly during the summer months/warmer areas for the flight crew to ensure there is enough fuel for the transport, equipment, and personnel traveling with the patient.
With some tank-based systems there is a need for a back-up injector module; accurate electronic gas delivery may not be available without it. Finally, a pre-use checkout which includes a purge procedure is required prior to transport with typical tank-based systems, requiring an additional 10 minutes prior to transport. A tankless system does not have these requirements and therefore can be much lighter in weight. Using other equipment off-label may require the addition of costly proprietary parts and pieces, and special training for respiratory therapist use on that improvised combination. A system approved by FDA and consistent with FAA regulations would be a valuable asset in transport on iNO.
Genosyl Delivery System: A System Vetted and Approved for Transport with iNO
Recognizing this value and the significance of almost 30 years of off-label use of iNO in the support of critically ill neonates during transport to a higher level of care, Vero Biotech (Atlanta, Georgia USA) was granted FDA approval on December 14, 2020, for use of Genosyl DS in the interhospital transport environment. The Genosyl device11 is the first tankless NODS, supplying iNO from 16-ounce cassettes instead of two six-pound D-cylinder tanks.11 The approval reflects rigorous testing and a careful design process. The Vero engineering team worked with key opinion leaders and transport experts in the field to design a transport mount that would reduce the overall system weight and footprint as much as possible.
The mount was designed to contour to the console geometry with minimal size and weight increase. A strap is utilized to allow easy console removal and replacement for set up. Helical mounts connected to the base of the transport mount provide shock and vibration dampening to allow the console to dose without disruption.
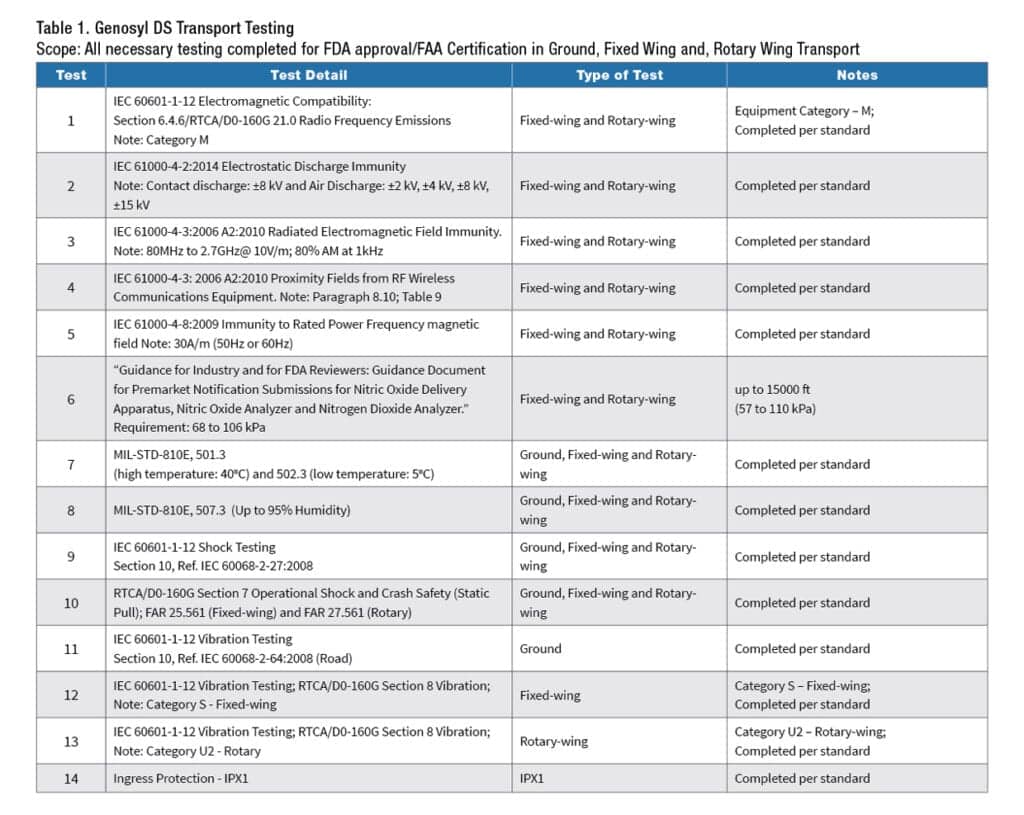
Applicable standards for crash safety, EMI/EMC, shock, and vibration were tested to ensure that the Genosyl DS and transport mount assembly can withstand the environments of ground, fixed-wing, and rotary-wing intrahospital transport. Table 1 outlines the suite of testing performed for the submission to the FDA.
Discussion
The value of “on-label” use of a drug or device is inclusive of several issues that focus primarily on patient safety. First, the indication means that the FDA has evaluated the safety and efficacy of the product for that specific use and found it appropriate for use in patients who have specific treatment needs. It does not mean uses outside the approved labeling are not safe, it simply provides assurance that the overall approved use has been reviewed specifically by the FDA.
Secondly, as alluded to above, it provides guidance and “guard rails” within which that safety and efficacy are assured. This is particularly beneficial for the inexperienced or only occasional user, to have one process or one dosing regimen approved and explained. Third, it offers consistent information on any monitoring that should be applied when the patient is using the device or drug. Finally, a FDA labeled indication offers a potential pathway to reimbursement for use, so that uninsured, underinsured, and insured patients can all have access to the drug or device, and providers such as hospitals or transport companies do not have to absorb the cost of the therapy.
FDA approval of the Vero transport NODS offers a streamlined approach to iNO use in acute care and in various transport settings. For those facilities that have adopted the Genosyl system, advantages include no need for training on new devices, the reassurance and time-saving of using an approved configuration, and an improved focus on patient safety for these vulnerable neonates as they are transported to higher levels of care.
RT
For more information, contact [email protected].
Author Affiliations
i Vero Biotech, Atlanta, Ga
ii Northwestern University Feinberg School of Medicine, Chicago, Ill
iii Ann & Robert H Lurie Children’s Hospital of Chicago, Chicago, Ill
iv University of Mississippi Medical Center, Jackson, Miss
Disclosures
- Mitchell, Crite, and Georgiev are employees of Vero Biotech.
- Pollack is a consultant to Vero Biotech.
- Compensation received by advisory board attendees (including author Newmyer) is reported in compliance with Sunshine Act and other regulatory requirements.
References
- Steurer MA, et al. Persistent Pulmonary Hypertension of the Newborn in Late Preterm and Term Infants in California. Pediatrics. 2017;139(1). doi:10.1542/peds.2016-1165
- Kinsella JP, et al. Recommendations for the Use of Inhaled Nitric Oxide Therapy in Premature Newborns with Severe Pulmonary Hypertension. J Pediatr. 2016;170:312-314. doi:10.1016/j.jpeds.2015.11.050
- Barrington KJ, et al. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2017;1:CD000399. doi:10.1002/14651858.CD000399.pub3
- How Many Hospitals Are in the US? Accessed January 6, 2021. https://blog.definitivehc.com/how-many-hospitals-are-in-the-us
- Dartmouth Atlas of Neonatal Intensive Care. Neonatal Intensive Care. 124.
- Gien J, et al. Inhaled Nitric Oxide in Emergency Medical Transport of the Newborn. NeoReviews. 2020;21(3):e157-e164.
- Lowe CG, Trautwein JG. Inhaled nitric oxide therapy during the transport of neonates with persistent pulmonary hypertension or severe hypoxic respiratory failure. Eur J Pediatr. 2007;166(10):1025-1031.
- Kinsella JP, et al. Inhaled nitric oxide treatment for stabilization and emergency medical transport of critically ill newborns and infants. Pediatrics. 1995;95(5):773-776.
- https://nasemso.org/wp-content/uploads/ACCT-Standards-Version1-Oct2016.pdf
- https://www.camts.org/wp-content/uploads/2019/07/2020_-12th-Edition-Stds-Second-Draft-with-highlighted-changes.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202860s000lbl.pdf