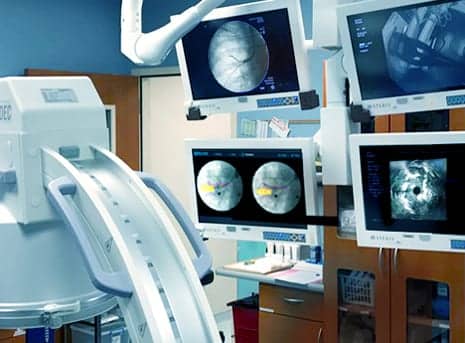
Body Vision Medical has received clearance from the US FDA to market LungVision, a novel imaging system that enables accurate real-time navigation and lesion localization during bronchoscopic procedures.
LungVision uses an “augmented reality” approach to allows physicians to plan, visualize, and track endobronchial tools and radiolucent lesions in real time. The system’s synergistic imaging merges intraoperative fluoroscopy with pre-operative high resolution imaging, such as computed tomography.
“Body Vision developed unique technology that combines the intelligence of navigation with the simplicity of fluoroscopy”, says D. Kyle Hogarth, MD, FCCP Associate Professor of Medicine, Director of Bronchoscopy at the University of Chicago and one of the first LungVision users in North America. “It enables effective localization and enhanced biopsy of lung lesions, upgrading the standard methods used in our daily practice”.
The first clinical experience with the LungVision System will be presented by Sean Stoy, MD, University of Chicago Medical Center, at the American Thoracic Society meeting on May 21, 2017 from 2:15-4:15 pm. The presentation is part of the rapid poster discussion session A110, “Advances of the thoracic oncologic diagnostics.”