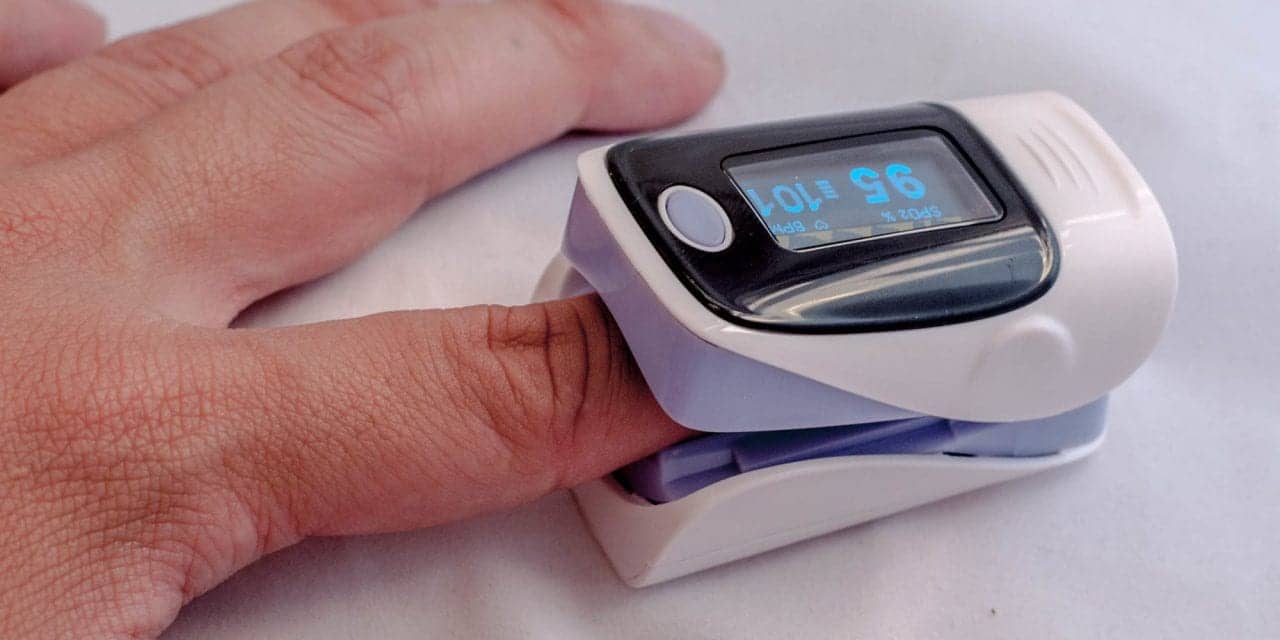
The FDA will convene a public meeting of its Medical Devices Advisory Committee later this year to discuss evidence about the accuracy of pulse oximeters, according to the agency.
The meeting is the result of ongoing concerns that these products may be less accurate in individuals with darker skin pigmentations”, the FDA said in a media advisory.
A recent study published in JAMA Internal Medicine found that non-white COVID patients were delayed care — or in some cases did not receive care — due to inaccuracies in pulse oximetry readings. The study compared patients’ ABG test results to the results of pulse oximeters and found that pulse oximetry overestimated blood oxygenation in racial and ethnic minorities, specifically overestimating blood oxygen levels by 1.2% for Black patients, 1.1% among non-Black Hispanic patients and 1.7% for Asian patients.
The agency said it “continues to evaluate all available information pertaining to factors that may affect pulse oximeter accuracy and performance.”
The committee will discuss the available evidence about the accuracy of pulse oximeters, recommendations for patients and health care providers, the amount and type of data that should be provided by manufacturers to assess pulse oximeter accuracy, and to guide other regulatory actions as needed, the agency said.
The FDA issued recommendations for taking pulse oximetry readings in February 2019 and emphasized those recommendations have not changed.
Additional details will be announced in the coming weeks.