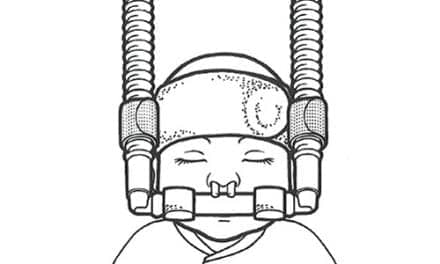
The US FDA has granted commercial clearance for the Cerêve Sleep System, a prescription device that reduces latency to Stage 1 and Stage 2 sleep for people with insomnia.
The Cerêve System is comprised of a software-controlled bedside device that cools and pumps fluid to a forehead pad that is worn through the night. Clinical subjects found the device easy to use and to wear, and commented that it was a calming and comfortable experience.
Functional brain imaging studies confirmed that the frontal cortex, or executive brain, stays active in people with insomnia during sleep, preventing them from getting deeper, more restorative sleep. The solution: gently cooling the forehead within a precise, clinically-proven therapeutic range in order to reduce this activity in the frontal cortex.
FDA Clears Cerêve Device for Insomnia