The key role played by an ancient protein in the course of idiopathic pulmonary fibrosis has been uncovered by scientists, according to new research that offers more than an unprecedentedly detailed explanation of the disease’s tragic course.
The new study features a central figure: an evolutionarily ancient protein called “chitinase 3-like-1” (CHI3L1). The authors implicate it as the “master regulator” of what appears to be a tragically errant repair response to the mysterious lung injuries that give rise to the disease. In describing how CHI3L1 works in IPF, the research also points to a strategy for treatment.
The report demonstrates that CHI3L1 is produced to help in response to the injury. It feeds back to protect injured cells from dying and simultaneously stimulates tissue repair to patch the damage that has occurred.
But the study also shows how this dual role contributes to the ultimate problem. If IPF resulted from a single injury, like a paper cut, CHI3L1 would decrease the injury and cause local scarring while it restored tissue integrity. In that case, the amount of scarring would not be excessive and tissue function would not be significantly altered.
But in IPF lungs, cells undergo ongoing injury, so CHI3L1 is chronically elevated and scar tissue accumulates. As CHI3L1 rescues cell after cell, the scarring builds up, eventually compromising the lung’s ability to breathe. In IPF, 70 percent of patients die within five years.
“The CHI3L1 is doing exactly what it is supposed to do — it is designed to shut off cell death and decrease injury,” said Jack A. Elias, a co-senior author of the study and dean of medicine and biological sciences at Brown University. “But at the same time it is decreasing cell death it is driving the fibrosis. You’ve got this ongoing injury so you’ve got these ongoing attempts to shut off injury which stimulate scarring.”
“This research lays the foundation for potential therapies that would be designed to diminish injury and ameliorate fibroproliferative repair.” Credit: Scott Kingsley for Brown UniversityIn patients and the lab
The research team, including co-senior author Erica Herzog of Yale and co-lead authors Yang Zhou (who is transitioning to Brown from Yale), Huanxing Sun of Yale, and Hong Peng of Central South University in China used various means to uncover CHI3L1’s central role in IPF.
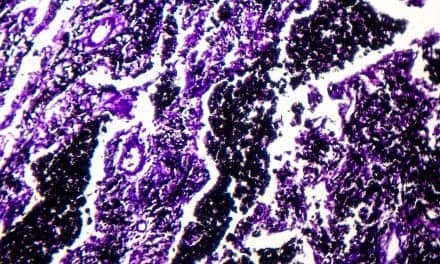
They compared tissues and serum from normal patients, outpatients with IPF, and patients with an acute exacerbation (AE) of IPF. In AE, widespread lung injury is superimposed on the pulmonary fibrosis, which frequently occurs before patients die. In lung biopsies and serum, they found that CHI3L1 levels are elevated in both tissue compartments in the outpatients with IPF and that the levels of CHI3L1 correlated with their disease progression. In the patients with AE, elevated levels of CHI3L1 were not noted, showing that the levels of CHI3L1 decrease right before the patients die.

The protein CHI3L1 works to protect injured cells and to repair damage. In lung tissue, damage repair means a buildup of scar tissue, which compromises the lung. Levels of CHI3L1 are much higher in patients with idiopathic pulmonary fibrosis (right) than in healthy controls. Credit: Brown University/Yale University
“This demonstrates that the CHI3L1 plays a key role in controlling lung injury in this setting,” Elias said.
After documenting that elevated levels of CHI3L1 correlate with ongoing fibrosis and scarring and that a lack of the protein associates with widespread cell death, the team engaged in several manipulations of CHI3L1 in mice to see how levels and the clinical outcomes might be related. (In mice, CHI3L1 is also called BRP-39.)
Scientists can induce an IPF-like response in mice using a drug called bleomycin. In mice given bleomycin, the researchers found that the levels of CHI3L1 declined at first and then surged. At the times when the protein levels were low, cell damage occurred, and when the protein surged, the excessive scarring set in.
In all, the results show that CHI3L1 plays a fundamental role in the course, if not the origin, of IPF. An ongoing buildup of it results in excessive scarring. Too little and cells die much more frequently.
“To my knowledge this is the first comprehensive paper that’s been able to explain the many facets and presentations of IPF,” Elias said. “It explains and links the injury and the repair responses that are critical in the disease. It also provides an explanation for the slowly progressing patients and the patients that experience acute exacerbations.”
Elias said he hopes the insights will lead to new therapies for IPF. The idea would be to preserve the cell-protecting function of CHI3L1, while tempering its ability to stimulate tissue scarring and repair.
There may indeed be a way to do that, Elias said. Some data suggest that the mechanisms for each CHI3L1 function – cell protection and tissue repair – involve different pathways and or receptors. In people, therefore, separate drugs could hypothetically enhance the injury prevention pathway and temper the repair pathway. Indeed, drugs that block a key repair pathway receptor exist and are undergoing testing in other diseases, Elias said.
“This research lays the foundation for potential therapies that would be designed to diminish injury and ameliorate fibroproliferative repair,” Elias said.