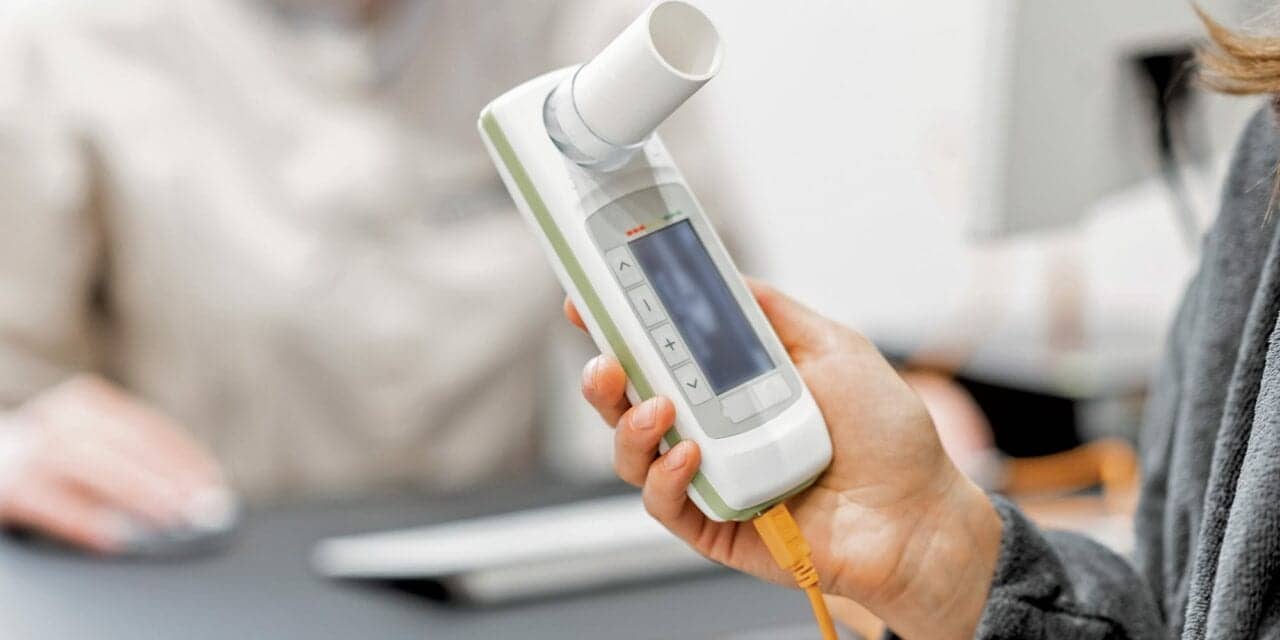
Objective tools that are useful in making a diagnosis and in management of asthma include spirometry and fractional exhaled nitric oxide (FeNO). However, there are complications and challenges in obtaining patient data with the changes brought by the COVID-19 pandemic.
By Bill Pruitt, MBA, RRT, CPFT, AE-C, FAARC
Asthma is often a challenging disease to diagnose and to treat. While sometimes there are clear test results and distinctive signs and symptoms that lock in the diagnosis, there may be times where things are not so clear. The diagnosis is confounded by age (asthma is often more difficult in children) or underlying comorbid conditions (such as Asthma-COPD Overlap Syndrome or ACOS), plus the intermittent appearance of symptoms. Some patients will have a flare up of symptoms that resolve over time and then have periods of no symptoms.
Likewise, treatment strategies may be straightforward and effective in many cases, while other times it is difficult to find the right strategy due to the many factors that can affect the outcome, from poor patient adherence to the plan, to lack of resources, healthcare disparities, to social or environmental influences, and more.
Asthma is both underdiagnosed (patients who have asthma but have not had it diagnosed) and over diagnosed (patients have been diagnosed with asthma but actually do not have the disease).1 Objective tools that are useful in making a diagnosis and in management of asthma include spirometry and Fractional exhaled nitric oxide (FeNO). However, there are complications and challenges in obtaining patient data with the changes brought by the COVID-19 pandemic. This article will explore the usefulness of these tools in working with patients who may (or may not) have asthma and touch on some considerations regarding the COVID-19 issues.
Spirometry
Spirometry has been a good initial tool to look for asthma and provide follow up after starting therapy. Advances in technology have enabled many patients to use spirometry to monitor/manage their disease at home and share the results with their provider. The 2020 update of the Global Initiative for Asthma (GINA 2020) includes spirometry in the diagnostic criteria for asthma and gives guidelines for three groups: adults, adolescents, and children age 6 to 11 years old.2
The first of the two diagnostic features is that there is a history of variable respiratory symptoms (including cough, wheezing, shortness of breath, and chest tightness). The patient may only have one symptom but more often two or more are present. Symptoms are variable over time and may be more or less intense, often may appear more at night, and are often triggered by exercise, strong emotions, exposure to allergens, or exposure to cold air. Viral infections will also tend to increase the appearance or intensity of symptoms.
The second diagnostic feature in GINA 2020 is confirmation of variable expiratory airflow limitation – with the confirmation resting on spirometry results. Airflow limitation is uncovered during a time where the forced expiratory volume in the first second (FEV1) is reduced. For suspected asthma, initial findings that helps confirm a diagnosis show the FEV1/Forced vital capacity (FVC) below 75-80% in adults and <90% in children combined with a subsequent increase >12% and >200 mL from the baseline in FEV1 in adults (or >12% predicted in FEV1 in children) within 10 to 15 minutes after receiving an albuterol treatment. The post- bronchodilator change in expiratory airflow is the basis of describing asthma as reversible. Reversibility can be blunted or absent in the face of severe exacerbation or viral infection, and in untreated asthma the airflow limitation can become fixed (or irreversible).2
Unfortunately, spirometry is often not being utilized in the diagnosis or management of asthma. A recent publication from 2020 in the Journal of Asthma and Allergy found that: “Among patients who had a diagnosis of asthma in 2014 and an asthma-related hospitalization or ED visit in 2016-2019, the majority had never had spirometry performed in the 2007-2015 study window and had never seen a specialist.”3 Office spirometry has been reviewed and evaluated in many studies. A device can recoup the cost of equipment in a relatively short period so cost should not preclude purchasing this equipment. Formal training of the personnel and ongoing education along with checks on quality of the testing is needed to obtain reliable test results.4
In suspected asthma the baseline findings may be normal (no airflow limitation happens to be present) so a subsequent test may be used to help uncover airway hyperresponsiveness. Often this involves bronchial provocation testing using either an exercise challenge test or a bronchial challenge test. In either of these tests, a positive result seen in a significant drop in FEV1 from baseline helps rule in the diagnosis of asthma. Subjects with normal airways—having no hyperresponsiveness (and who are not currently taking controller medications), will not demonstrate a significant drop in FEV1 with these challenges.
GINA 2020 also includes variability of peak expiratory flow (PEF) as part of the second diagnostic feature where twice-daily PEF measurements over a two week period show >10% variability in adults and >13% variability in children. However, GINA 2020 also states that FEV1 from spirometry is more reliable that PEF.2
Spirometry use in the home is a growing trend that helps patients and providers monitor asthma in the home environment. Advances in technology and connectivity has led to the availability of several handheld, portable, electronic spirometers that can allow patients to perform tests, check the quality of the test, monitor their airway function, and have this information relayed to their healthcare provider via smartphone or computer. As noted in a 2018 article reviewing 16 of these devices, the researchers state: “Regular spirometry testing can result in earlier detection of exacerbations, quicker recovery times, earlier treatment, reduced health costs, and an overall improved quality of life.”5 More studies are being performed and published concerning the use of home-based spirometry.
With the advances in technology (such as Bluetooth connectivity for devices such as portable spirometers, MDI and nebulizer remote tracking, and wearable devices such as a smart watch), home-based monitoring is gaining ground and increase the shift from office-based to home-based management of asthma care. In light of the COVID-19 pandemic, gathering reliable patient data remotely reduces the need for coming into a healthcare center thus reducing possible exposure to both the patient and healthcare professionals.6-8
Fractional Exhaled Nitric Oxide (FeNO)
FeNO has gained strength in helping diagnose and monitor/manage asthma cases. This biomarker is described as an indirect marker for the extent of airway inflammation.9 FeNO can be detected with devices on the market by either chemiluminescence analyzers or electrochemical sensors but research is looking into use of laser technology and smart solid-state microsensors.10
Regarding its use as a diagnostic tool, a systematic review of published articles concluded that FeNO provided a fairly accurate measure for making a diagnosis and, given that since the specificity of the test was higher than the sensitivity, it tends to have a higher diagnostic potential to rule in rather than rule out asthma.9 Measurement of FeNO should be accompanied by a questionnaire to determine if confounding factors need to be considered (such as genetics, sex, weight, height, diet, smoking, or use of anti-inflammatory medications).10
FeNO is also being evaluated in looking for particular asthma types such as cough-variant asthma and occupational asthma.11 In the management of asthma, use of FeNO has been shown to reduce exacerbations. It may have a role in managing asthma during pregnancy, in assessing patient compliance and in titrating corticosteroid dosage. Researchers are also investigating FeNO monitoring to assess effectiveness of the dupilumab (an anti-IL-4/-13 therapy) and sublingual immunotherapy against house dust mites.11
GINA 2020 mentions FeNO several times throughout the document. At this time the guidelines do not recommend using FeNO to guide treatment but states that “further studies are needed to identify populations most likely to benefit from FeNO-guided treatment and the optimal frequency of FeNO monitoring.”2
GINA 2020 describes several studies that included FeNO in the data gathered which shows the usefulness of FeNO as a part of the objective data in researching treatment strategies, medications, and asthma phenotypes. FeNO has also been used to assess patient adherence to the use of inhaled corticosteroids. For example, GINA 2020 mentions one study where five days of direct observation therapy suppressed previously high FeNO levels and indicated poor adherence on the part of the patient.
FeNO may gain ground in monitoring children with asthma as more evidence is published in this area. A Cochrane review published in 2016 concluded that FeNO monitoring “significantly decreased the number of children who had one or more exacerbations over the study period but did not impact on the day-to-day clinical symptoms or inhaled corticosteroid doses”.
The authors went on to say: “Further randomized controlled tests need to be conducted and these should encompass different asthma severities, different settings including primary care and less affluent settings, and consider different FeNO cut-offs.”12
COVID-19 Issues
The COVID-19 pandemic brought major changes in healthcare as strategies were put in place to reduce an overwhelming surge in exposures, infection, hospitalizations and deaths. Routine diagnostic testing including pulmonary function testing (PFT) was suspended for months in light of the potential for these procedures to produce aerosols and coughing, thus increasing the risk of transmitting the disease to both the public and to healthcare professionals.13 Testing in the PFT laboratory has resumed in limited capacity. The American Lung Association has published a brief comprehensive guide to help do this testing as safely as possible.14
Their recommendations include such items as:
- Limit testing to only cases that are absolutely necessary
- Careful prescreening on arriving at the site (including an assessment of patient symptoms and possible exposure, measuring their temperature) and delay testing for 30 days if warranted.
- Use of face masks by patient as much as possible, maintaining a minimum of 6 feet between patients, use of minimum 70% alcohol hand sanitizer prior to and after testing
- Staff should use PPE during the spirometry test, including: N95, face shield, gown, and gloves
- Thorough disinfection of all equipment and high touch surfaces before and after testing
- Keep doors closed except for entering and exiting
- Use of a negative pressure room if possible. Exhaust air from room directly to the outside of the building, wait between patients until air exchanges have occurred that clear the room (for rooms that have six air exchanges per hour, the wait between patients would be a minimum of 70 min).
- Use a filter on the spirometer circuit if possible
As mentioned earlier, portable electronic spirometers can provide an alternate to having asthma patients come to a PFT laboratories. The cost for these devices is relatively low, maintenance is simple, and use of these units at home to provide frequent measurements (weekly or even daily) adds a new and timely component to managing the disease remotely. In addition, home-based spirometry can reduce the demand on the central PFT laboratory and allow these sites to focus their limited resources on cases that are more urgent or establishing a new diagnosis.13
Conclusion
Spirometry is a major component in diagnosing and managing asthma, and FeNO is becoming an established means of monitoring and guiding patient care. Office spirometry needs to be more utilized with attention paid to proper training, ongoing education, and quality control. Home-based spirometry shows potential to change the way asthma is managed in the general scheme and is offering an alternative to centralized testing in light of the COVID-19 pandemic. FeNO is no longer a new, novel measurement and as research continues, it will likely become a mainstay in working with asthma patients.
Respiratory therapists who work with asthma patients need to be very familiar with these two procedures. This includes knowing the equipment, the meaning of the results, the standards for obtaining quality tests, and what needs to change to perform these procedures due to the impact of the COVID-19 pandemic. In addition, therapists need to recognize that when spirometry and/or FeNO data missing—they need to be advocates for the patient and recommend that the missing data be obtained and used to guide diagnosis and management of asthma.
RT
Bill Pruitt, MBA, RRT, CPFT, AE-C, FAARC, senior instructor emeritus, is a writer, lecturer, and consultant and recently retired from over 20 years teaching at the University of South Alabama in Cardiorespiratory Care. He also volunteers at the Pulmonary Clinic at Victory Health Partners in Mobile, Ala, providing respiratory care for uninsured adults. For more information, contact
[email protected].
References
- Aaron SD, et al. Underdiagnosis and overdiagnosis of asthma. American Journal of Respiratory and Critical Care Medicine. 2018 Oct 15;198(8):1012-20.
- Global strategy for asthma management and prevention. 2020. Accessed via www.ginasthma.org.
- Roychowdhury P, et al. Spirometry Utilization Among Patients with Asthma. Journal of Asthma and Allergy. 2020;13:193.
- Ruppel GL, et al. Office Spirometry in primary care for the diagnosis and management of COPD: National Lung Health Education Program Update. Respiratory Care. 2018 Feb 1;63(2):242-52.
- Carpenter DM, et al. A review of portable electronic spirometers: implications for asthma self-management. Current Allergy and Asthma Reports. 2018 Oct 1;18(10):53.
- Kruizinga MD, et al. Towards remote monitoring in pediatric care and clinical trials—Tolerability, repeatability and reference values of candidate digital endpoints derived from physical activity, heart rate and sleep in healthy children. Plos One. 2021 Jan 7;16(1):e0244877.
- Kupczyk M, et al. Home self-monitoring in patients with asthma using a mobile spirometry system. Journal of Asthma. 2020 Jan 6:1-7.
- Ferrante G, et al. Digital health interventions in children with asthma. Clinical & Experimental Allergy. 2020 Nov 25.
- Karrasch S, et al. Accuracy of FeNO for diagnosing asthma: a systematic review. Thorax. 2017 Feb 1;72(2):109-16.
- Heffler E, et al. FENO in the management of asthma: a position paper of the Italian Respiratory Society (SIP/IRS) and Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). Multidisciplinary Respiratory Medicine. 2020 Jan 28;15(1).
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Current Opinion in Allergy and Clinical Immunology. 2020 Feb 1;20(1):71-9.
- Petsky HL, et al. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database of Systematic Reviews. 2016(11).
- References 13-14 available at www.respiratory-therapy.com