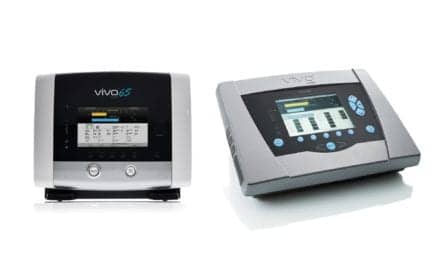
Dräger has received FDA clearance for its Seattle PAP (Positive Airway Pressure) plus system, which uses bubble CPAP technology to treat premature infants with respiratory distress. The device was developed by Seattle Children’s Research Institute and produced and marketed by Dräger.
The Seattle PAP plus leverages bubble CPAP technology and further improves upon it to make it easier for to breathe, according to Dräger.
Worldwide, nearly one million infants die annually from respiratory distress syndrome (RDS).1 In the US alone, there are approximately 18,000 hospitalizations each year due to RDS at a cost of approximately $2.3 billion.2
Although Bubble CPAP has been a treatment option for neonates for more than 40 years, there has always been a desire to further reduce the work of breathing for these patients and increase the safety of the therapy. Historically, a major complication of Bubble CPAP in preemies is respiratory muscle exhaustion, where the baby’s respiratory muscles wear out, which can result in respiratory failure.1
The design of the Seattle PAP plus addresses both of these concerns. The 135-degree angle of the bubbler increases the oscillations with the goal of offering more efficient respiratory support, lessening the work required by respiratory muscles and making it easier for babies to breathe.1,3 The second design difference is the locking mechanism for the pressure level, which avoids inadvertent changes of CPAP level and increases safety of the therapy delivered.
“Seattle Children’s Research Institute wanted a better option for treating respiratory distress in premature babies so we worked with them to bring a solution to the market that addresses a previously unmet need in the neonatal intensive care unit (NICU),” said Steve Menet, senior vice president of sales, hospital solutions, Dräger Inc. “This is a great example of how healthcare industry stakeholders can come together to address a clinical need to improve patient outcomes.”
The Seattle PAP plus is effective, safe and easy to use:
- Efficacy: The patented design is totally focused on the infant’s clinical needs, enabling the clinician to:
- Easily adjust CPAP from 4.5 to 10 cm H2O
- Quickly connect the Seattle PAP plus system to the appropriate BabyFlow plus patient interface
- Treat patients with the needed masks and prongs in combination with BabyFlow plus
- Safety: The system incorporates safety features to avoid inadvertent changes in the position of the tube and subsequent changes in CPAP levels, which can cause serious harm to the infant. To eliminate the risk of cross-contamination, all system components are single-patient use.
- Ease of use: A single kit provides all the parts needed to assemble the Seattle PAP plus therapy, allowing the caregiver to worry less about assembly and more about the patient.
This new product further solidifies Dräger’s commitment to noninvasive and invasive ventilation from neonates to adults, the company said in a press release.
- Short term evaluation of respiratory effort by premature infants supported with bubble nasal continuous airway pressure using Seattle-PAP and a standard bubble device. PLOS ONE, March 28, 2018, Stephen E. Welty et al.
- Respiratory Distress Syndrome of the Newborn, https://www.thoracic.org/patients/patient-resources/breathing-in-america/resources/chapter-19-respiratory-distress-syndr.pdf
- Bubble CPAP: is the noise important? An invitro study. Pediatr Res 2005;57(6):826-830. Pillow JJ, Travadi JN.