A startup company developing a video game-based pulmonary rehab device adapted its product into a home spirometer during the COVID-19 pandemic with the support of NIH/NHLBI funding.
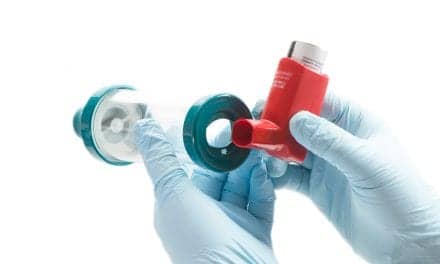
According to an NHLBI press release, the company, Zephyrx, developed a system that included an existing FDA-approved, handheld breathing device – about the size of a cell phone – and a newly created app called Breathe Easy. The company’s motivation was to solve the problem of patients with chronic lung diseases being unable to visit clinics and labs for PFT during the COVID-19 pandemic, NHLBI explained.
As patients began cancelling appointments and some medical offices closed, doctors needed a way to monitor those who could not be seen in person. And they needed to act fast.
Thomas Smith, MD, a pulmonary critical care physician at Albany Medical Center in New York, explained the problem to healthcare startup, Zephyrx. They were working with Smith on an NIH clinical trial involving a breath-activated video game for improving lung function in post-surgical patients. Zephyrx proposed that the therapeutic device be re-purposed as an at-home diagnostic tool for measuring lung function remotely.
“When no one wants to come in the hospital, how do I assess my patients who have advancing lung disease?” Smith asked. “All of a sudden, telemedicine made sense.”
The system functions much like a conventional spirometer, the device commonly used to measure lung function, in that it requires patients to blow into a tube to evaluate their breathing efficiency. But an office-based spirometer is relatively large and requires both the patient and technician to be present. The Zephyrx system is compact and allows patients to conduct pulmonary function tests at home on their own tablet or smartphone. It also has an in-app video call feature that allows the patients’ healthcare providers to communicate directly with them throughout the lung function test.
Dwight Cheu, CEO of Zephyrx, describes the system as a portable spirometry lab. “Our platform is able to extend a pulmonologist’s ability to treat patients wherever they are, whether they are four hours away or around the corner,” Cheu said. “We have the ability to treat people in this time of COVID and beyond COVID.” The device is easy to use, provides results in real-time, and works for a wide variety of different lung conditions, including COPD, ALS, asthma, as well as cystic fibrosis, he said. Based on the results, doctors can adjust medications and treatment accordingly.
Today, the Zephyrx Breathe Easy platform is used in over 500 clinics across the country. Recently, the Cystic Fibrosis Foundation purchased a device for every patient with cystic fibrosis in the United States – at least 20,000 people. Demand for the device is expanding nationwide, Cheu said.
The company developed the innovative technology with funding support from the NHLBI through its Small Business Innovation Research (SBIR) Program with significant assistance from the NIH’s Concept to Clinic: Commercializing Innovation (C3i) Program.