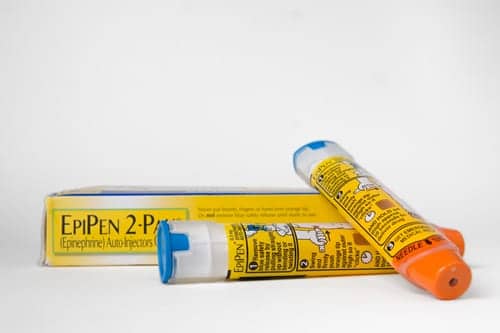
The makers of the EpiPen failed to properly investigate more than 100 complaints that the device malfunctioned during life-threatening emergencies — including situations in which patients later died, according to a Food and Drug Administration warning letter sent to a Pfizer company.
The FDA said in the letter that Meridian Medical Technologies, a Pfizer company that manufactures the auto-injectors used to treat life-threatening allergic reactions, had detected faults in some units of the EpiPen and had failed to adequately respond.
The Tuesday warning letter noted that the manufacturer had received 171 complaints about products that failed to activate between 2014 and 2017, but did not disassemble “the vast majority” of the units as part of its investigation. The device is designed to inject epinephrine to halt dangerous allergic reactions.
FDA: EpiPen Manufacturer Failed to Investigate Product Defects