RTs may find it difficult to choose between available inhaled pulmonary vasodilators (IPVs)—inhaled nitric oxide or nebulized epoprostenol—to treat pulmonary hypertension.
Acute pulmonary hypertension (PH) is a life-threatening condition that affects people of all ages. It is often treated in intensive care units where patients can be cared for by a team of clinicians, and since respiratory support is often required, respiratory therapists play a key role in patient management.1
There is currently debate in the respiratory field over the use of inhaled pulmonary vasodilators (IPVs), a class of inhaled drugs commonly used to treat these patients. RTs may find it difficult to choose between available IPVs—inhaled nitric oxide (iNO) and nebulized epoprostenol (iEPO)—in their effort to balance patient safety with cost-containment for their institution. These are high-risk decisions as patients with acute PH are often critically ill.
Very little has been documented about how RTs make such decisions. To gain insight into current practices, Mallinckrodt Pharmaceuticals engaged the American Association for Respiratory Care (AARC) to survey RTs on their use and perception of IPVs. To qualify for the survey, RTs had to indicate personally administering nebulized prostacyclin and inhaled nitric oxide to at least one patient in an average month (not necessarily the same patient). Of the qualified respondents, 34% (19/56) indicated administering inhaled prostacyclins to more than six patients in a typical month and 32% (18/56) administer iNO to six or more patients in an average month (data not shown).2
Background
Pulmonary hypertension can be defined as any condition that elevates the pulmonary arterial pressure (PAP) ?25 mmHg.3 High PAP occurs when blood flow is restricted through the lungs in response to conditions such as hypoxemia or a pulmonary embolism.1 The restricted blood flow causes back pressure on the right ventricle (RV), or RV afterload, which can lead to heart failure if left untreated.1,3
Inhaled pulmonary vasodilators have become the gold standard treatment for patients with acute PH requiring respiratory support. Vasodilators help reduce the elevated PAP and can relieve stress to the right ventricle. Compared with intravenous or oral administration of vasodilators that elicit a systemic effect, administration of vasodilators by inhalation concentrates their effects on the pulmonary vasculature. Systemic hypotension can reduce the flow of blood into the right ventricle and exacerbate RV failure.4 Therefore, inhalation of vasodilators delivers the drug to where it can be most effective while simultaneously reducing the chances of a potentially life-threatening side effect. The caveat with this method of administration is that any sudden withdrawal or interruption of IPV delivery can lead to a rapid recurrence of symptoms, otherwise known as rebound pulmonary hypertension, which can be potentially fatal.5-7
Inhaled NO and iEPO are two commonly used IPVs to treat acute PH. Each drug targets a different molecular pathway to relax the smooth muscle cells of pulmonary arteries. Relaxation of smooth muscle cells promotes vasodilation, which can reduce PAP and decrease the RV afterload.8 Inhaled NO and iEPO can also support oxygenation by improving V/Q (ventilation/perfusion) matching in the lungs.6 Since iNO and iEPO are both capable of reducing PAP and improving oxygenation in patients with acute PH, the use of one IPV over the other is often influenced by safety.
Safety Considerations When Assessing an IPV
There are numerous factors that influence IPV safety. Most revolve around ensuring that IPV delivery is sustained throughout the course of therapy, from initiation to weaning. IPV administration can last from several hours to several days. Any abrupt discontinuation or insufficient levels of drug can cause rebound PH.5-7 In addition, bolus doses or high concentrations of iNO or iEPO can cause systemic hypotension and toxicity.7,9 Administration of too much or too little drug can be life-threatening. This is why the integration of the IPV device into the ventilation circuit, adequate staffing for monitoring delivery, and the presence of back-up or redundant systems, are all essential for the safe administration of iNO and iEPO to patients with acute PH.
IPV delivery systems differ in their safety features, set-up, and staffing requirements. Inhaled NO has a dedicated delivery system, the INOmax DSIR, which is FDA-approved for use with patients requiring respiratory support. It has been validated for use with over 60 ventilators and noninvasive respiratory support devices.10 Inhaled epoprostenol is commonly administered using the Aerogen Mesh nebulizer. While this nebulizer is FDA-cleared to deliver drugs approved for inhalation, the delivery of epoprostenol from this device is considered “off-label” because most forms of epoprostenol are not FDA-approved for inhalation.9
To ensure drug delivery is not interrupted in the event of a power loss, the INOmax DSIR and Aerogen Mesh nebulizer each have a back-up power supply.11-12 Although 55% of RTs in the survey report that delay or interruption of drug delivery due to device failure (power interruption, malfunction, etc.) rarely occurs (see Table 1, in pink), 70% of respondents agree that it is unsafe to administer IPVs using devices that lack an internal back-up power supply. (See Table 2, in gold.)
RTs feel that back-up drug delivery systems are also important for preventing interruptions in drug delivery, with 61% of RTs in the survey agreeing that it is unsafe to administer IPVs using devices that lack back-up delivery options (see Table 2, in gold). Only the INOmax DSIR has a back-up delivery mode.11
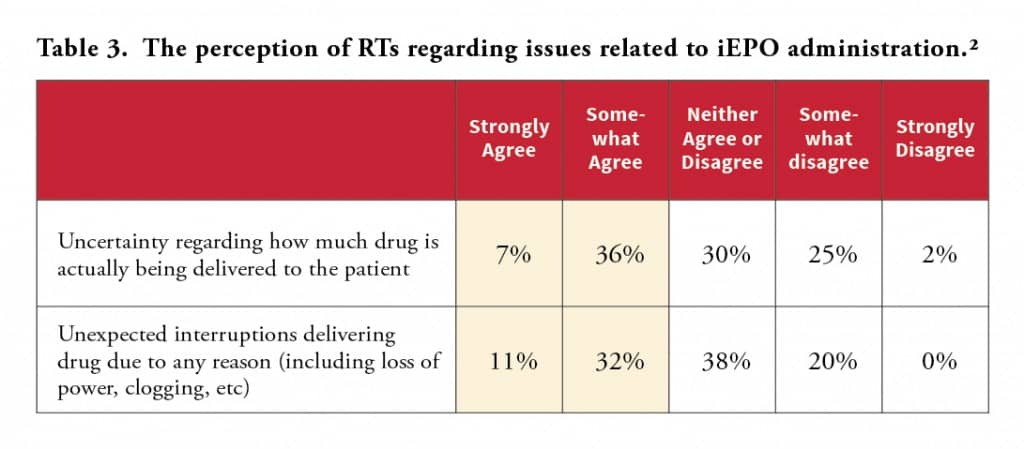
While RTs in the survey reported that incidents in which a patient requiring an unplanned manual ventilation due to an interruption in drug delivery, or has drug delivery interrupted or stopped due to device failure, are rare, they do occur (see Table 1, in pink). Forty-three percent of respondents in the survey view unexpected interruption of drug delivery for any reason as a significant problem in their practice (see Table 3, in gold).
Each IPV delivery device must provide a steady dose of drug over the course of treatment. Many RTs in the survey view uncertainty regarding how much drug is actually being delivered to the patient (43%) as a significant problem with administering iEPO (see Table 3, in gold). According to results, 82% of respondents agree that it is unsafe to administer IPVs using devices that lack delivery failure alarms. In addition, 51% agree that it is unsafe to administer IPVs using devices that lack drug concentration alarms (see Table 2, in gold).
When nebulizing epoprostenol into a ventilator circuit, filters must be placed on the expiratory limb to prevent the solution from clogging the ventilator.13
RTs in the survey reported that interruption or discontinuation of drug delivery due to drug delivery issues occurred more frequently with iEPO (44% total—5% frequently, 39% occasionally) than with iNO (12% total—5% frequently, 7% occasionally). (See Table 1, in pink.) Respondents reported more monitoring by staff with iEPO (56%) than iNO (27%). (See Table 1, in pink.) Moreover, 81% of the RTs agree that safe administration of iEPO requires hourly patient bedside checks to ensure drug delivery has not been interrupted (see Table 2).
Awareness Among RTs
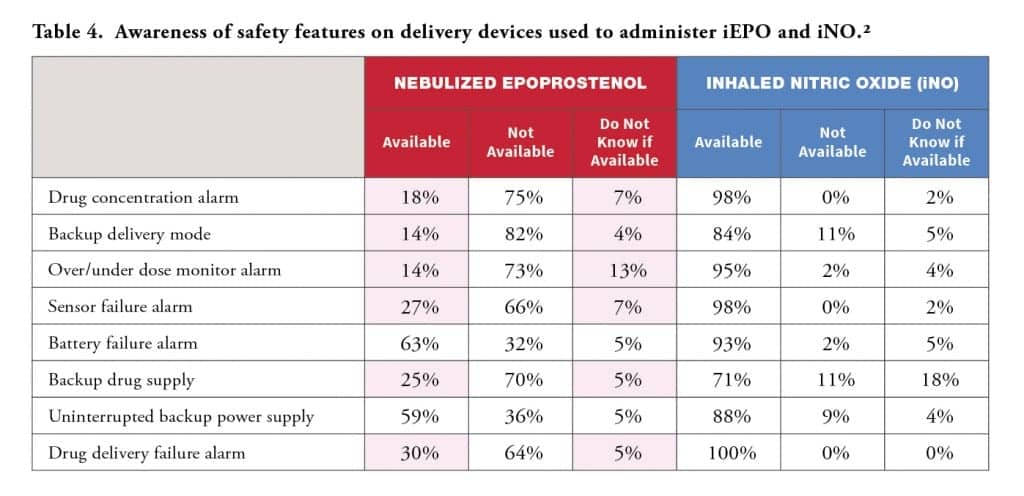
RTs agree that IPV delivery devices should have back-up delivery options, delivery failure alarms, drug concentration alarms, and an internal back-up power supply (see Table 2). While most RTs in the survey have a clear understanding of the safety features on the INOmax DSIR, they are less familiar with the features on nebulizers. Between 18%-34% of respondents did not know if a feature was available or they incorrectly reported the availability of a feature on iEPO nebulizers (see Table 4, in pink).
The survey also revealed that most of the RTs in the cohort have a limited awareness of the pH of the IPVs they administer. Fifty-nine percent admitted to not knowing the pH of INOmax (iNO, pH=7), 52% indicated not knowing the pH of Flolan (epoprostenol, pH=10.2-10.8), and 54% indicated not knowing pH of Veletri (epoprostenol, pH=11-13).14-15 Only 20% and 16% knew the correct pH of Flolan and Veletri, respectively (data not shown).2 While additional research needs to be done, one animal study reported mild tracheitis secondary to inhalation of a high pH buffer.16 It is important for RTs to be aware of unresolved safety concerns associated with the drugs they administer, especially when these concerns are not openly discussed in the community.
One issue that prevents RTs from having a clear picture of IPV safety is the pervasive underreporting of device-related problems and concerns. Sixty-eight percent of respondents in the survey agree this underreporting of device-related problems and concerns is noteworthy (data not shown).2 In a poll of the audience at the 2015 AARC Congress on IPV device safety, 93% of respondents believe that clinicians under-report alarm-related events.17 This may be partly because RTs may be unfamiliar with reporting requirements and mechanisms.
All incidents where a device causes or contributes to the death or serious injury of a patient should be reported to the FDA and posted on their Manufacturer and User Facility Device Experience (MAUDE) database. Device manufacturers and device user facilities (including hospitals, outpatient diagnostic or treatment facilities, nursing homes and ambulatory surgical facilities) are required to submit a medical device report (MDR) if they become aware of an incident.
While several hundred thousand MDRs are submitted to the MAUDE database each year, this is a passive surveillance system and events often go unreported.18 RTs can directly report such incidents to device manufacturers or to the FDA. Often, RTs use internal channels at their institutions to report incidents, but what is not clear is what percentage of these reports reach the device manufacturers or the FDA.
A Call to Action
Like many decisions that critical care clinicians face, IPV assessments are fraught with concerns and risks. The best way to make life-or-death decisions is to be well-informed. Unfortunately, the use of IPVs to treat acute PH is mostly done off-label, and clinical trial data only supports the use of one of these drugs (iNO) in a specific infant population with a specific type of PH (near-term and term neonates with persistent pulmonary hypertension, or PPHN).7
It is unlikely at this juncture that the pharmaceutical companies that produce nitric oxide or epoprostenol will fund a head-to-head non-inferiority study to compare these drugs and solidify their efficacy and safety in other patient populations. Therefore, the only way RTs can obtain large scale safety data is by making sure that the incidents they report are posted on the MAUDE database. Word-of-mouth and experience are important when making a clinical assessment, but these methods cannot surpass the power of a comprehensive dataset. Every incident that goes unreported is a missed opportunity to warn others of a potential hazard.
RTs can support each other’s IPV assessments by becoming more diligent in reporting safety issues. Each reported incident is a learning opportunity not only for the clinicians who administer the drug, but for the device manufacturers who are constantly trying to improve their delivery devices. When an incident goes unreported, device manufacturers lose an opportunity to learn about an issue occurring with their device in the field. When the community shares its knowledge and experience, everyone benefits.
Conclusion
The management of acute PH can be challenging. The survey of 56 RTs who regularly administer both iEPO and iNO has yielded several insights into drug, alarm, safety, and monitoring concerns. RTs are at the forefront of current clinical practice, and advances in treatment are often crafted at the bedside. RTs can foster innovation and ensure patient safety by diligently reporting incidents to device manufactures and/or the FDA using internal channels within their organizations and external channels with the assistance of their organizational leadership.
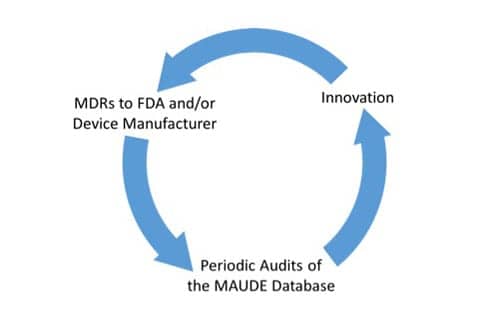
Device manufacturers are continually assessing MDRs, and utilize these incidents to guide improvements in their products. To ensure safe and effective clinical practice, RTs are encouraged to review the MAUDE database on a regular basis to acquire information about IPV administration. Learning from these MDR audits can be used to guide and improve their clinical practice:
In the end, everyone will benefit from this cycle of report-based innovation; the most important beneficiary being the patient. RT
Garry W Kauffman, RRT FAARC, MPA, FACHE, is manager of Kauffman Consulting, LLC. For more information, contact [email protected].
References
- Hoeper MM, Bogaard HJ, Condliffe R et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013; 62(25 Suppl):D42-50.
- Data on file. Mallinckrodt Pharmaceuticals, Mallinckrodt PLC.
- Hui-li G. The management of acute pulmonary arterial hypertension. Cardiovasc Ther. 2011; 29(3):153-175.
- Lowson SM. Alternatives to nitric oxide. Br Med Bull. 2004; 70:119-131.
- Augoustides JG,Culp K, Smith S. Rebound pulmonary hypertension and cardiogenic shock after withdrawal of inhaled prostacyclin. 2004; 100(4):1023-1025.
- Walmrath D, Schneider T, Schermuly R, et al. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996; 153(3):991-996.
- INOmax Prescribing Information. Hampton, NJ. Mallinckrodt Pharmaceuticals. 2013.
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004; 351(14):1425-1436.
- Siobal MS. Aerosolized prostacyclins. Respir Care. 2004; 49(6): 640-652.
- INOmax DSIR and DSIR Plus Ventilator Validation List (US). Hampton, NJ. Mallinckrodt Pharmaceuticals. 2015. http://inomax.com/wp-content/uploads/2015/01/DSIR_DSIRPlus_Ventilator_Validation-0515.pdf.
- Guidance Document for Premarket Notification Submissions for Nitric Oxide Delivery Apparatus, Nitric Oxide Analyzer and Nitrogen Dioxide Analyzer. 2000. http://www.fda.gov/downloads/MedicalDevices/…/ucm073767.pdf.
- http://www.aerogen.com/products/aerogen-solo.html.
- De Wet CJ, Affleck DG, Jacobsohn E, et al. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2004; 127(4): 1058-1067.
- FLOLAN Prescribing Information. GlaxoSmithKline, Inc. 2011.
- VELETRI Prescribing Information. Actelion Pharmaceuticals US, Inc. 2012.
- Van Heerden PV, Caterina P, Filion P, et al. Pulmonary toxicity of inhaled aerosolized prostacyclin therapy—an observational study. Anaesth Intensive Care. 2000; 28(2):161-166.
- [Link to AARC monograph – in preparation]
- FDA Maude Database. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed April 23, 2015.