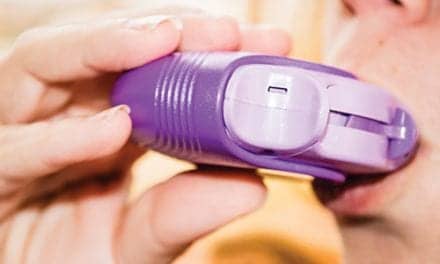
D R Burton Healthcare Products LLC has launched the iPEP Oscillating Positive Expiratory Pressure (OPEP) device, which uniquely combines OPEP and Incentive Spirometer therapies to address all-three respiratory needs: lung expansion, treatment of atelectasis, and secretion clearance.
The iPEP system includes a removable, palm-sized PocketPEP, a compact OPEP device for active patients for use at home to help prevent pulmonary-related readmissions.
“The iPEP system represents a breakthrough in airway secretion clearance therapy by providing patented volumetric-based OPEP therapy. The iPEP takes the mystery out of OPEP therapy by providing patients and healthcare providers with biofeedback that measures the patient’s inspiratory capacity during therapy,” according to Dennis Cook, BSRT, RRT, CEO of D R Burton.
Along with a slow coached inhalation, the iPEP provides superior expiratory flow bias, a key driver of secretion clearance outlined in a recently published study.
Cook explains, “There is a need to prevent pneumonia in high risk patients, such as postoperative and patients with increased mucous production. The D R Burton family of OPEP products are designed to reduce the risk of not only hospital acquired pneumonia, but also reduce risk of pneumonia at home after discharge.”
D R Burton will showcase the device at the ATS 2017 from May 19-24 in Washington DC, and at the National Teaching Institute & Critical Care Exposition on May 22-25 in Houston.