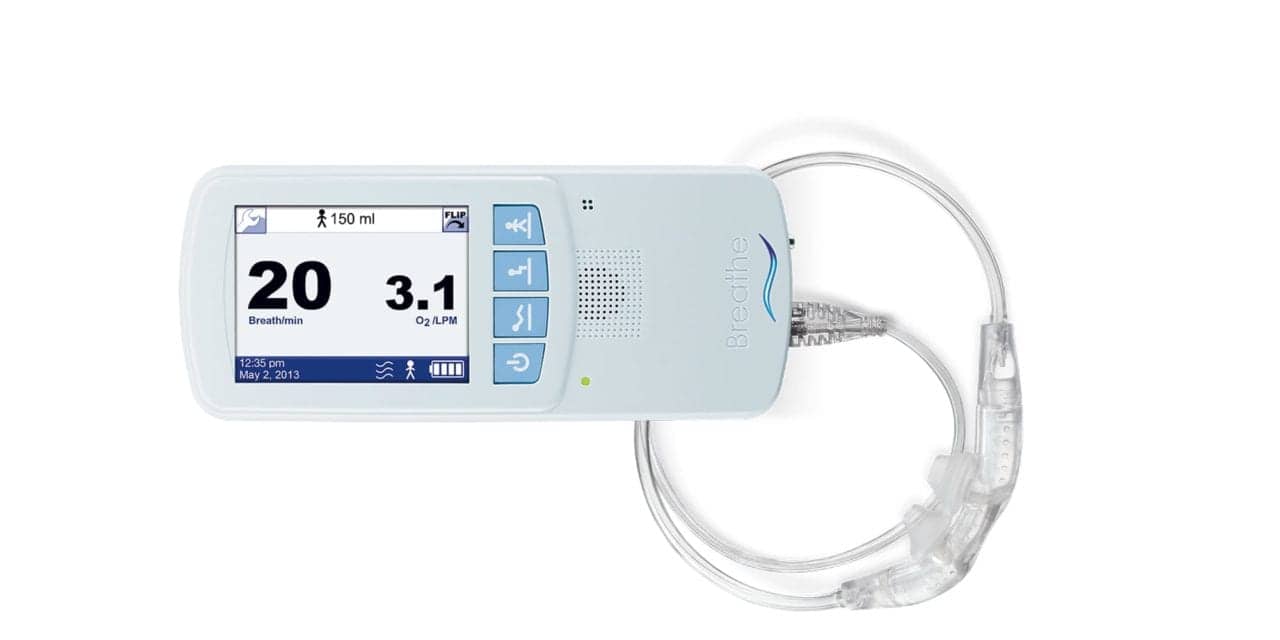
Baxter International Inc has issued an Urgent Medical Device Correction for the Hillrom Life2000 Ventilation System, the FDA reports. According to an FDA alert, the correction is due to the potential for patient oxygen desaturation events that can occur under certain conditions when the Life2000 system is connected with a third-party oxygen concentrator.
The FDA warns that oxygen desaturation could occur if:
- hoses that are kinked or have excessive moisture;
- modified, extended or loose/disconnected tubing;
- oxygen liter flow from the concentrator that has fallen below the prescribed level while using the Life2000 system;
- and/or non-compliance with recommended cleaning and maintenance of the Life2000 system and oxygen concentrator.
Baxter has received reports of patient desaturation that required hospitalization. Based on analysis to date, no deaths have been reported related to this issue, the FDA reports.
This Urgent Medical Device Correction applies to the following: all Life2000 Ventilation Systems used with an oxygen concentrator, including:
- the Life2000 Ventilator Packaged (BT-20-0002);
- the Life2000 Ventilator Packaged A (BT-20-0002A);
- the Life2000 System AC Package (BT200007);
- the Breathe Life2000 Ventilator PA (BT-20-0007);
- the Life2000 System HC Package (BT200011);
- the Breathe Technology Life2000 VE (BT-20-0011); and
- the Life2000 Ventilator V6.X (MS-01-0118).
The FDA says patients can continue to use the Life2000 system if they follow daily checks and preventive maintenance requirements as detailed in the patient letter and Instructions for Use for both the Life2000 Ventilation System and third-party oxygen concentrators. These actions will help ensure the best oxygen delivery with the Life2000 system when used with a third-party oxygen concentrator.
Read more at the FDA website here.