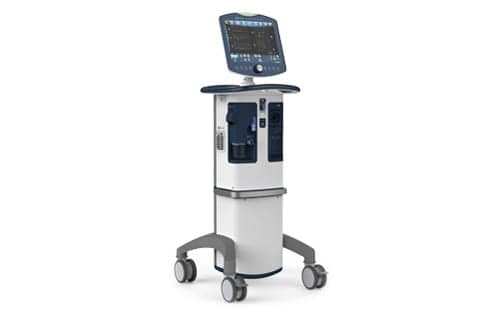
Covidien, LP (part of Medtronic) issued a Puritan Bennett 980 Ventilator recall due to a capacitor manufacturing assembly error that may cause the ventilator to become inoperable or stop working as intended, according to an FDA alert.
If this occurs, it could result in the loss of ventilation and serious adverse events such as hypercarbia, hypoxemia, neurological injury or death.
There have been six complaints and one death regarding this device issue. There have been no reported injuries.
RECOMMENDATIONS
On November 4, 2021, Medtronic sent an “Urgent Medical Device Correction” letter to customers requesting:Identify affected devices and discontinue use of these devices.
Remove the affected devices from clinical service and quarantine those ventilators until such time that a Medtronic Technical Service Engineer can inspect and replace the affected printed circuit board assemblies.
Notify all personnel in all care environments in which the affected PB980 series ventilators are used about this medical device correction.
If your facility has distributed affected PB980 series ventilators to other persons or facilities, please promptly forward a copy of this letter to those recipients.
Complete the Urgent Medical Device Correction form included with the letter and return it as directed to confirm your receipt and understanding of this information.If you are aware of any incidents related to this issue, please contact the Medtronic Technical Support Department immediately at 1-800-255-6774, option 4, and then option 1 to provide information regarding those events so regulatory reporting obligations can be fulfilled.Work with the Medtronic Technical Support Department if you require assistance finding alternative ventilation devices.
Health professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:Complete and submit the report online.Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on form, or submit by fax to 1-800-FDA-0178.