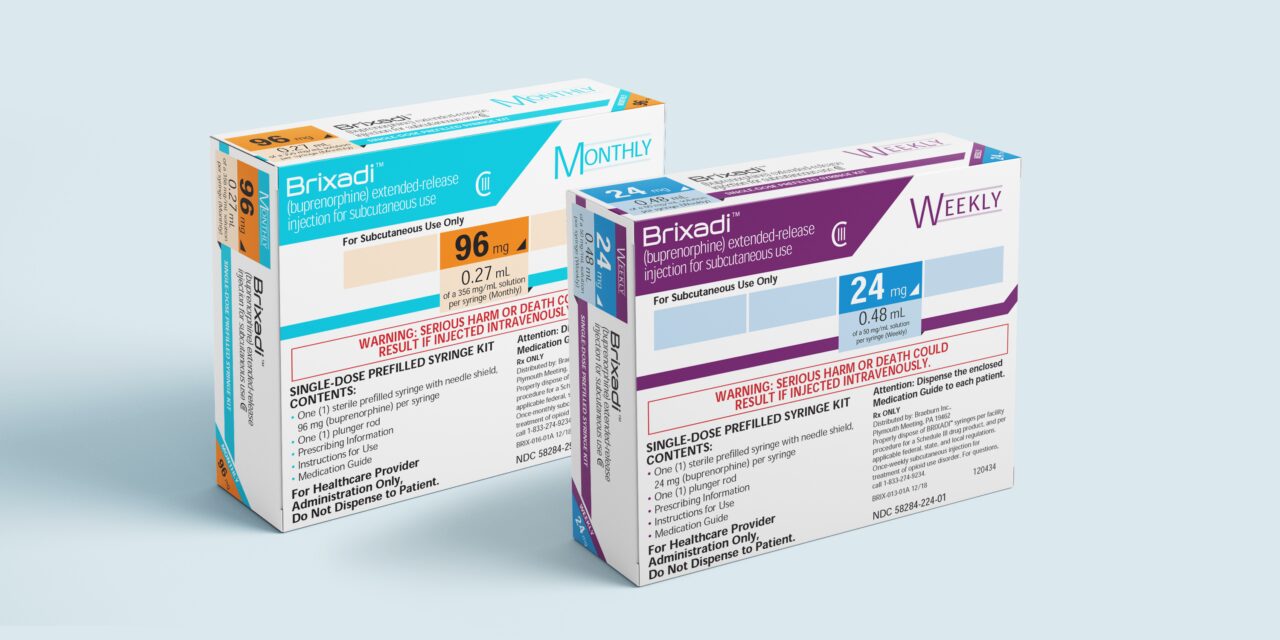
The US Food and Drug Administration (FDA) approved Camurus’ Brixadi (buprenorphine) extended-release injection for subcutaneous use to treat moderate to severe opioid use disorder.
Brixadi is available in two formulations, a weekly injection that can be used in patients who have started treatment with a single dose of a transmucosal buprenorphine product or who are already being treated with buprenorphine, and a monthly version for patients already being treated with buprenorphine.
“Buprenorphine is an important treatment option for opioid use disorder. Today’s approval expands dosing options and provides people with opioid use disorder a greater opportunity to sustain long-term recovery,” says FDA commissioner Robert M. Califf, MD, in a release.
Brixadi is approved in both weekly and monthly subcutaneous injectable formulations at varying doses, including lower doses that may be appropriate for those who do not tolerate higher doses of extended-release buprenorphine that are currently available. The weekly doses are eight milligrams, 16 milligrams, 24 milligrams, and 32 milligrams; and the monthly doses are 64 milligrams, 96 milligrams, and 128 milligrams.
The approved weekly formulation in various lower strengths offers a new option for people in recovery who may benefit from a weekly injection to maintain treatment adherence, according to a release from the FDA. Brixadi will be available through a Risk Evaluation and Mitigation Strategy program and is to be administered only by health care providers in a health care setting.
“A weekly and monthly buprenorphine injection with different dose options can align with clinical practice and patient care needs,” says Michelle Lofwall, MD, professor of behavioral science and psychiatry at the University of Kentucky Center on Drug and Alcohol Research and primary investigator in the phase 3 efficacy and safety study, in a release. “Some patients need help with taking their medication as prescribed; some prefer not taking a daily medication or visiting a pharmacy to pick up their medication. So having a weekly and monthly option that provides buprenorphine over one week or one month could benefit patients, their loved ones, and the treatment providers.”
The safety profile of Brixadi was consistent with the known systemic safety profile of oral buprenorphine with the exception of mild to moderate injection-site reactions. The most common adverse reactions included injection-site pain, headache, constipation, nausea, injection-site erythema, injection-site pruritus, insomnia, and urinary tract infections.
Brixadi will be available in the US in September. The product will be marketed in the US by Camurus’ licensee Braeburn.
Photo credit: Braeburn