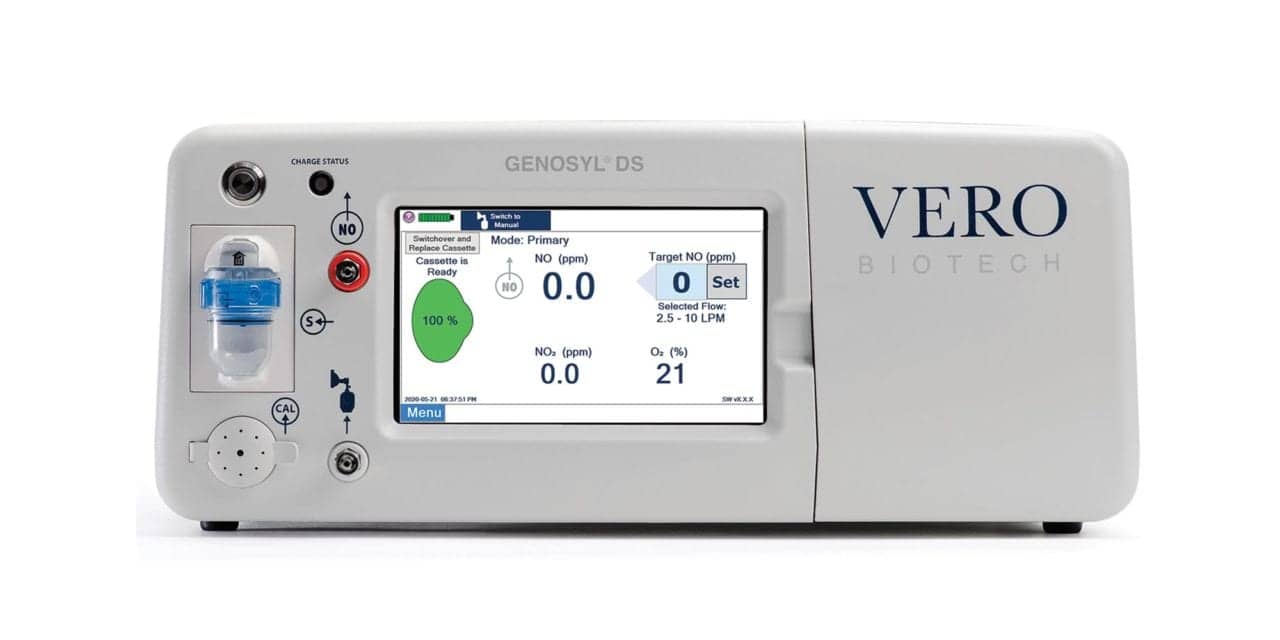
Vero Biotech Inc announced FDA approval of its second-generation Genosyl tankless inhaled nitric oxide (iNO) delivery system for use with rebreathing anesthesia in the operating room setting.
The advantages of rebreathing anesthesia have made this method the standard of care for anesthesia administration in the operating room setting, according to the company, which says its second-generation delivery system is now the first and only device for iNO delivery that is approved for use in both rebreathing and non-rebreathing anesthesia methods.
The expected benefits of the second-generation system as approved for rebreathing anesthesia include the following:
- Ability to use rebreathing anesthesia: lower gas flows, i.e., less use of costly anesthetic agents and savings for the hospital; increased patient comfort (by preserving patient body temperature and moisture)
- Streamlined process of care: seamless iNO delivery from the intensive care unit through surgery to post-operative care, resulting in process and workflow improvements for the health care organization that reduce overall cost to the hospital
- Set and forget: allows anesthesiologists to continue to use rebreathing anesthesia and therefore could prevent potentially dangerous, cumbersome, and time-consuming workarounds
- Reduced environmental impact of anesthesia delivery: reduction in release of anesthetic to the environment
“We believe this new indication for our second-generation device will now provide significant benefits to the anesthesiology and surgical care communities whose patients require inhaled nitric oxide in the operating room setting,” says Brent V. Furse, CEO and president of Vero Biotech, in a press release. “We have addressed an unmet need in facilitating rebreathing anesthesia, a further demonstration of our continuous commitment to neonatal intensive care and the acute care hospital community in providing solutions to the challenges they face.”
It is important to note the FDA approval of the Genosyl delivery system for use with rebreathing anesthesia in the operating room setting is for the second-generation device. The recently approved third-generation Genosyl delivery system has not been tested with rebreathing anesthesia. Vero Biotech is currently conducting similar validations and expects to have the data available this quarter.