With COVID-19 compromising the diagnosis and management of respiratory diseases such as COPD in hospitals, is it time to break free from the PFT lab?
Georg Harnoncourt, CEO of NDD Medical Technologies
The global COVID-19 pandemic has had a tremendous impact on healthcare systems, causing issues including hospital overcrowding, disruptions to services and increased risks for patients and healthcare workers alike.
As both healthcare organizations and patients try to reduce the number of visits to hospitals to only the necessary, there is now a growing risk to those with respiratory diseases such as chronic obstructive pulmonary disease (COPD)—both in terms of delayed diagnosis and disease management—due to reduced access to pulmonary function testing (PFT) services from lung function labs. In addition, many COVID-19 patients themselves will also require safe and accessible follow-up testing to monitor recovery, putting an additional strain on labs and raising questions of safety and viability.
It is becoming clear that under the current circumstances, the traditional PFT set-up, usually performed in hospital-based lung function testing laboratories with large, cumbersome and static equipment, lacks the flexibility to easily adapt to this changing landscape.
The COVID-19 crisis does however, present a unique opportunity to re-think how PFT is performed and to ask if this could be improved upon. A possible solution to the current crisis could be to move PFT out of these laboratory settings and to the point of care (POC)—in settings such as physician offices, at clinics or at the bedside. This would help to restart services during the pandemic and, when used in combination with extra safety measures such as inline filters, it would enable safe and accessible monitoring of patients recovering from COVID-19.
However, there are wider and longer lasting benefits of moving PFT to the point of care, not least the potential for earlier diagnosis of respiratory diseases—which raises the question, is it time to leave the lab? Here, we look into current methods of PFT and note how the current pandemic situation is providing a perhaps overdue opportunity to reassess how respiratory diseases are diagnosed and managed in the future.
Are Laboratories the Fastest Route to Diagnosis?
In order to answer this question let’s take a closer look at PFT using COPD as an example. According to the World Health Organization, more than 3 million people die each year from COPD1, equating to an estimated 6% of all deaths globally and making it the third leading cause of death worldwide2. However, despite these alarming statistics, over 50% of people living with COPD remain undiagnosed3.
One of the main reasons for this is lack of access to testing facilities. Even in developed countries lung function labs can be few and far between, compounded by the fact that many patients are not referred to the lab until the condition has progressed, often because comorbidities like cardiac disease, diabetes mellitus, and hypertension can mask COPD, making early diagnosis challenging.
In order to make diagnoses, lung function laboratories provide complete PFT, including spirometry, diffusing capacity of the lung for carbon monoxide (DLCO), and absolute lung volumes. The labs usually base equipment on and around body plethysmography systems or ‘body boxes’—large, static equipment used primarily for the assessment of lung volumes (Figure 1).
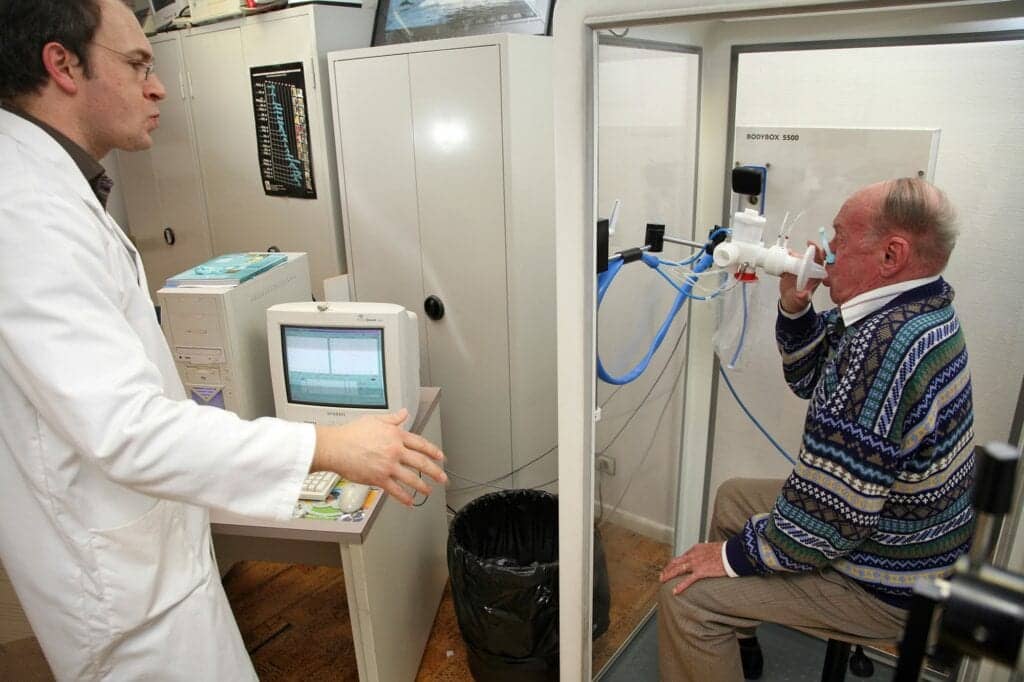
While spirometry is regarded as the gold standard for diagnosing COPD4, DLCO, which is a quantitative measure of gas transfer from the lungs to the blood, has been shown to be the most powerful predictor of survival in patients with COPD5. It is used to pinpoint the type of respiratory disease when an obstruction or lung volume issues are predetermined, and this fact makes DLCO in combination with spirometry one of the most valuable tools for diagnoses of COPD.
DLCO is therefore, a critical component of PFT that helps medical professionals provide a more accurate diagnosis of COPD, which allows for better treatment, patient-centric care and enables patients to live a higher quality of life.
However, while spirometry systems on their own are generally widely accessible in primary care settings such as occupational health, DLCO has only traditionally been available in lung function labs, which as discussed earlier, are not numerous and many patients are only referred to later in disease. Therefore, making complete PFT testing including DLCO accessible to patients and healthcare professionals earlier, in primary care practices, has the vast potential to streamline healthcare provision, provide more and earlier diagnoses and, most importantly, to improve patient outcomes.
The Time Is Right for Point of Care PFT
With the COVID-19 pandemic continuing to evolve, the need to perform complete PFT testing including DLCO quickly and accurately outside of the hospital environment has never been greater, and the advantages of switching PFT to the point of care are abundantly clear. In addition to dramatically reducing the number of hospital visits, point of care PFT would save lives by diagnosing COPD earlier, thereby also identifying those at greater risk of contracting COVID-19 and enabling shielding measures to rapidly be put in place.
Fortunately, putting PoC testing into practice should be relatively simple, since the necessary technology is already available. A new generation of highly precise, fully automated and maintenance free compact PFT instruments—the EasyOne product line from NDD Medical Technologies—has been designed specifically for this need. Such systems are fully compliant with American Thoracic Society (ATS)/ European Respiratory Society (ERS) standards and specific country regulations, and provide a reliable and portable solution for complete PFT including DLCO, spirometry, lung volumes, multiple-breath washout (MBW) and lung clearance index (LCI) with no need for the traditional body boxes (Figure 2). Devices such as these are set to make accurate, consistent and reliable lung function testing easier and more accessible.

As well as being a game-changer for COPD diagnosis and disease management, point of care PFT will also be vital in monitoring the recovery of COVID-19 survivors. With institutions such as Johns Hopkins reporting that those who recover from the disease may have long-term issues with their lung function and health6, point of care PFT will play a central role in guiding patients through the COVID-19 recuperation process. Portable PFT devices such as the aforementioned EasyOne systems have the added benefit of being inherently resistant to contamination by design, as breath does not come into direct contact with the sensors. In addition, optional inline filter solutions provide a double layer of COVID-19 protection for patients and healthcare professionals alike.
As we adapt to a world beyond COVID-19, and the incidence of COPD and other comorbid diseases continues to rise, solutions such as these portable PFT devices will be key tools in switching essential lung function testing to a more accessible and adaptable way of working—without sacrificing on accuracy and reliability for confidence in results. This will translate to faster and improved diagnoses, improved quality of care and patient outcomes and reduced healthcare costs. And with the portable technology already available for PFT, perhaps it’s time to break free from the constraints of the lab and think outside the (body) box.
RT
Georg Harnoncourt is CEO of NDD Medical Technologies, a leading provider of PFT equipment dedicated to the early detection and diagnosis of chronic lung diseases.
References
- www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_2
- Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom. 2018; 3: e4
- Tilert T et al. Prevalence and factors associated with self-reported chronic obstructive pulmonary disease among adults aged 40-79: the National Health and Nutrition Examination Survey (NHANES) 2007–2012. EC Pulmonol Respir Med. 2018; 7(9): 650–662.
- https://goldcopd.org/gold-spirometry-guide/
- Boutou AK et al. Lung function indices for predicting mortality in COPD. European Respiratory Journal 2013 42: 616-625.
- www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/what-coronavirus-does-to-the-lungs









