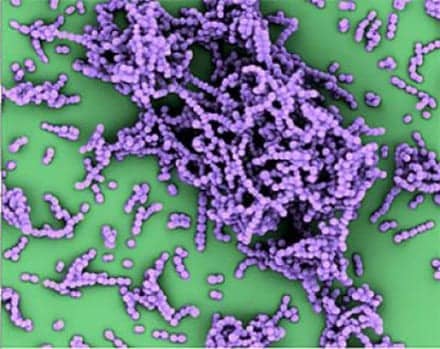
This is an electron micrograph, in false color, of group A Streptococcus bacteria.
Credit: UC San Diego School of Medicine
An international team of scientists, led by researchers at the University of California, San Diego School of Medicine, have identified the genes encoding a molecule that famously defines Group A Streptococcus (strep), a pathogenic bacterial species responsible for more than 700 million infections worldwide each year.
The findings, published online in the June 11 issue of Cell Host & Microbe, shed new light on how strep bacteria resists the human immune system and provides a new strategy for developing a safe and broadly effective vaccine against strep throat, necrotizing fasciitis (flesh-eating disease) and rheumatic heart disease.
“Most people experience one or more painful strep throat infections as a child or young adult,” said senior author Victor Nizet, MD, professor of pediatrics and pharmacy. “Developing a broadly effective and safe strep vaccine could prevent this suffering and reduce lost time and productivity at school and work, estimated to cost $2 billion annually.”
Efforts to develop such a vaccine have been significantly hindered by complexities in how the human immune system reacts to the bacterial pathogen. Specifically, some patients with strep infections produce antibodies that cross-react with their own heart valve tissue, leading to rheumatic fever and heart damage. Though rare in the United States, rheumatic fever remains common in some developing countries and causes significant disability and death.
The Cell Host & Microbe study suggests a way to circumvent the damaging autoimmune response triggered by strep. Specifically, the researchers noted that the cell wall of strep is composed primarily of a single molecule known as the group A carbohydrate (or GAC) which, in turn, is built from repeating units of the bacterial sugar rhamnose and the human-like sugar N-acetylglucosamine (GlcNAc).
Previous research has indicated that GlcNAc sugars present in GAC may be responsible for triggering production of heart-damaging antibodies in some patients. Nizet said the latest findings corroborate this model, and suggest that eliminating the pathogen’s ability to add GlcNAc sugars to GAC could be the basis for a safe vaccine.
“In this study, we discovered the strep genes responsible for the biosynthesis and assembly of GAC, the very molecule that defines the pathogen in clinical diagnosis,” said first author Nina van Sorge, PharmD, PhD, a former postdoctoral fellow at UC San Diego who now leads her own laboratory at Utrecht University Medical Center in the Netherlands. “This discovery allowed us to generate mutant bacterial strains and study the contribution of GAC to strep disease.”
Researchers plan to assess the new modified antigen against other candidates in advanced strep throat vaccine tests in nonhuman primates beginning later this year in Atlanta, Georgia, funded by the National Health and Medical Research Council of Australia.
“It is satisfying to find that a fundamental observation regarding the genetics and biochemistry of the pathogen can have implications not only for strep disease pathogenesis, but also for vaccine design,” Nizet said.