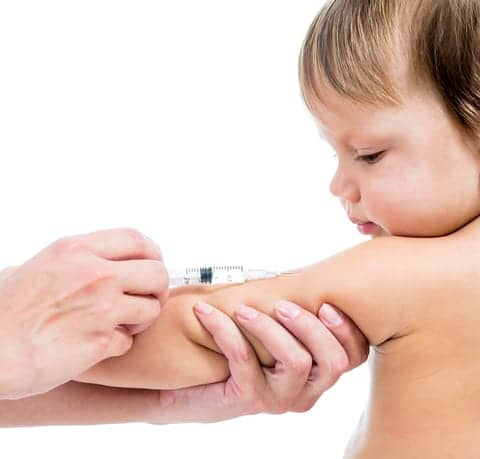
FluLaval, a vaccine that inoculates against Influenza A subtype viruses and type B viruses, has now been approved by the FDA for use in children as young as six months old. It had previously been approved for people aged three years and older.
Before approval of an expanded age indication for FluLaval quadrivalent, providers who preferred prefilled syringes had to order and stock two separate influenza vaccines in order to be able to immunize all patients, the company notes in a news release.
“With this approval, providers are now able to use the same dose of FluLaval quadrivalent (15 ?g of hemagglutinin per virus strain in 0.5 mL) to vaccinate all recommended persons aged 6 months and older.”
Approval of the supplemental biologics license application for FluLaval in children as young as 6 months of age was based on one phase 3 pivotal study and three supportive clinical studies conducted in children aged 6 months through 35 months, the company said.
Learn more at www.medscape.com