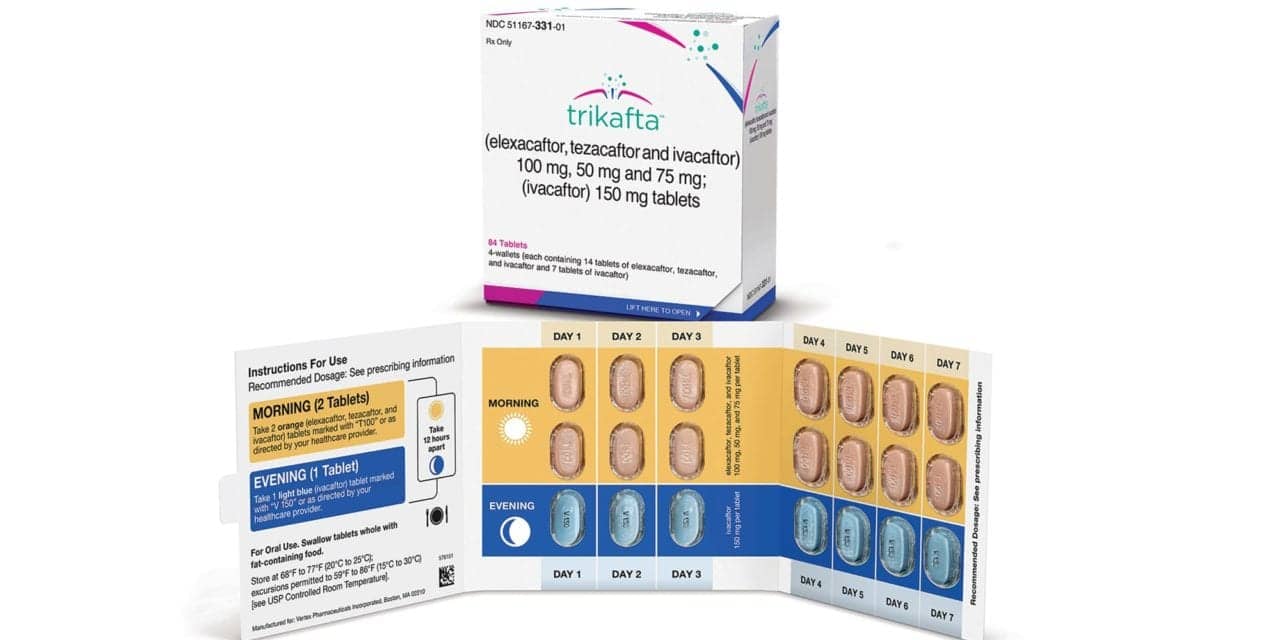
The FDA has approved the use of the Trikafta therapy to treat cystic fibrosis (CF) in children aged 6 to 11, making the therapy the first approved treatment for CF in the age group, according to an announcement from Vertex Pharmaceuticals.
Trikafta, approved in 2019 to treat patients 12 years and older, is expected to reach about 1,500 newly eligible children in the United States, the company said in a statement on Wednesday.
The treatment, currently priced at $311,503 per year, targets a defective protein responsible for the rare, life-threatening disease.
CF is caused by a mutation of the CFTR gene, which regulates salt and water content in cells. A protein defect leads to mucus congestion in the lungs and the digestive tract.
“Once a child with CF gets a pulmonary exacerbation, their lung function rarely recovers to pre-exacerbation levels, so this new ability to treat them early is really key,” said Andrew Fein, an analyst at HC Wainwright.