COPD patients treated with Targeted Lung Denervation (TLD) experienced a 50% reduction in COPD symptoms compared to patients who received a sham treatment, according to the AIRFLOW 2 phase II clinical trial.
[maxbutton id=”1″ url=”http://info.respiratory-therapy.com/regform” text=”SUBSCRIBE TO NEWS” ]
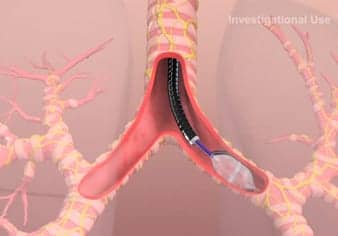
TLD involves passing a special catheter through an instrument called a bronchoscope into the lungs. The bronchoscope allows doctors to see the inside of a patient’s airways and to use the catheter to deliver a type of electrical charge called radiofrequency energy to the nerves on the outside of the airways, interrupting their normal function. This causes the airways to relax and widen, decreases mucus production and decreases airway wall inflammation.
TLD targets a well-known pathway involved in the development of COPD, called the cholinergic pathway, which regulates the body’s inflammatory responses to injury and promotes smooth muscle constriction of the airways. Anti-cholinergic drugs, which are often used to treat COPD, also target this pathway, but when they are combined with TLD there appears to be an additional, beneficial effect, even in patients who are being heavily treated with other drugs.
The AIRFLOW 2 clinical trial is taking place in several centers in six European countries. Patients are randomized to receive either TLD or a sham procedure under general anesthetic. The sham procedure still involves inserting the bronchoscope and catheter, but the radiofrequency electrical change is not delivered. This is performed under “double-blind” conditions, in which the treatment team that is carrying out the procedure knows whether the patient is assigned to the active treatment or the sham, but the follow-up team who sees the patient for post-procedure visits and tests does not, and nor does the patient.
Patients in both parts of the trial received tiotropium, an anti-cholinergic bronchodilator.
According to results presented at ERS 2018, 82 patients were enrolled in the trial. Three to six months after the treatment, 71% of patients who had received the sham treatment had an adverse respiratory event related to COPD compared to 32% of patients who received the TLD treatment.
“We have been able to significantly reduce chronic respiratory symptoms such as shortness of breath, exacerbations of the disease, infections and hospitalizations in a group of COPD patients who are already on aggressive medical therapy,” said principal investigator Dr Dirk-Jan Slebos of the University Medical Centre Groningen (The Netherlands). “There was also a trend towards improved quality of life and better lung function in the treated patients. This has important implications for patient quality of life, and also healthcare costs, as these are events that have a significant impact on the cost of caring for these patients.”
“Furthermore, the positive benefit has continued in those receiving TLD treatment, with the number of patients hospitalized for respiratory complications in the first year reduced by more than half in the treatment arm versus the sham arm,” said Slebos.
None of the patients died and there were no TLD-related adverse side effects that needed to be treated. Five patients (12%) who received the TLD treatment experienced stomach problems such as nausea, abdominal bloating and digestion discomfort, but these were temporary symptoms that had gone after six months.
“These occurred because of the radiofrequency energy affecting the nerves lining the nearby gullet,” said Slebos. “We are improving our process and imaging in order to better understand where these gastric nerves are, and we have implemented additional measures to improve our ability to avoid them in future procedures.”
A larger, phase III trial, AIRFLOW 3, is now being planned. It is likely to be launched in carefully chosen centers of excellence in Europe, beginning some time next year.
“These are really exciting and important results,” said professor Daiana Stolz, chair of the European Respiratory Society Education Council who was not involved in the study. “COPD is a difficult disease to treat successfully and the results from this well-conducted trial of Targeted Lung Denervation show that it can make a significant difference to the health of patients. Better treatments for COPD patients, particularly those with severe disease, are desperately needed and we look forward to the results from the AIRFLOW-3 trial, which we hope will confirm this as an effective and safe treatment.”