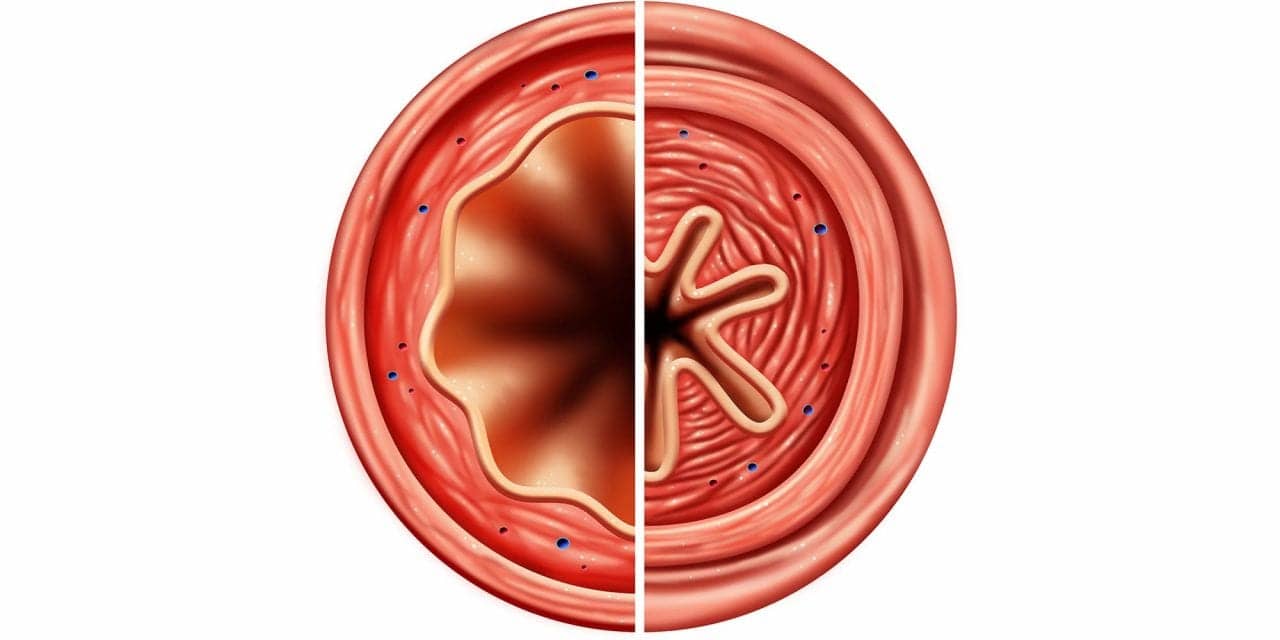
Endocannabinoids—cannabinoids produced within the body—are part of a signaling pathway that dilates the airways, according to new research published by scientists at Ruhr University in Germany. Researchers also believe deficiencies in these endocannabinoids may be one of the causes of asthma.
“Asthma is an inflammatory process, but what is fatal is the constriction of the bronchial tubes,” said Annika Simon, lead author of the study. “This is why we are very much interested in the regulation of this constriction.”
According to findings published in Nature Communications, researchers report that the endocannabinoid anandamide (AEA) induced prominent airway relaxation in vitro and in vivo. “Since our results show that anandamide dilates the bronchial tubes, we wanted to understand the exact mechanism behind it,” explained professor Daniela Wenzel, from the Department of Systems Physiology at Ruhr.
Researchers say the two best-known receptors for anandamide (CB1 and CB2) are irrelevant for this regulation. Therefore, there must be an alternative signaling pathway through which the messenger substance anandamide acts on the bronchial tubes.
Wenzel and her team showed that this alternative pathway uses an enzyme called fatty acid amide hydrolase (FAAH). FAAH degrades anandamide, producing arachidonic acid, which in turn is converted to prostaglandin E2. “We know that prostaglandin E2 can dilate the bronchial tubes,” explained researcher Annika Simon. Prostaglandin E2 acts via certain receptors and leads to an increase in the messenger substance cAMP (cyclic adenosine monophosphate).
“It is precisely this, the increase in cAMP, that is targeted by well-established inhalation medications against asthma,” said Wenzel. So, the goal is the same, but the path is different.
Wenzel and her team gradually deciphered the signaling pathway. They revealed that the enzyme FAAH is located both in the smooth muscle of the bronchial tubes and in the ciliated epithelium. The increase in cAMP after anandamide administration could be detected both in the mouse model and in human bronchial cells.
In order to find out whether anandamide could also work in asthma patients, the team used a disease model in mice where certain substances can be used to create artificial asthma. In these animals, too, the administration of anandamide led to a widening of the bronchial tubes. “This means that asthma doesn’t result in resistance to anandamide,” explained Wenzel.
Moreover, the researchers found that asthmatic animals have less anandamide and other endocannabinoids in their bronchial system than healthy animals. “Therefore, it’s possible that this anandamide deficiency is one of the causes of bronchial asthma,” concluded Wenzel.
The discovery of the new signaling pathway could also open up new possibilities for intervening in the disease process. “But there’s still a long way to go, and it will certainly take several years,” said Wenzel.
She expressly warned patients not to undertake experiments with cannabis plants.
“We can’t draw any direct conclusions regarding plant cannabinoids from the findings on endogenous cannabinoids. Exactly which other ingredients are found in cannabis plants besides the known cannabinoids is entirely unclear. Plus, the plants sometimes contain harmful substances.”
Nevertheless, the findings of this study are already pointing towards a better understanding of the body’s own cannabinoid system, which could lead to new treatment options for lung diseases in a few years’ time.